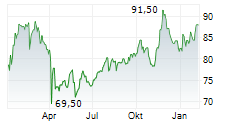
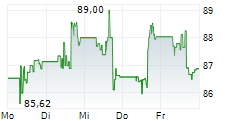
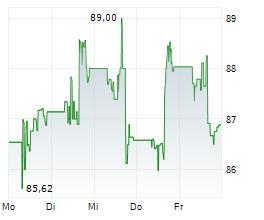
WASHINGTON (dpa-AFX) - Healthcare technology company Medtronic plc (MDT) announced Tuesday that the U.S. Food and Drug Administration (FDA) has granted two regulatory milestones for its MiniMed 780G system. The agency cleared the SmartGuard algorithm as an interoperable automated glycemic controller (iAGC), allowing integration with Abbott's Instinct sensor for type 1 diabetes, and approved the MiniMed 780G system for use in adults 18 and older with insulin-requiring type 2 diabetes.
'These milestones mark an important next step in our work to bring the proven performance and outcomes of our MiniMed 780G automated insulin delivery system to more people living with diabetes. By enabling integration with the Instinct sensor and expanding the MiniMed 780G system to people with type 2 diabetes, we are advancing a smart dosing ecosystem designed to provide greater choice and flexibility, along with a more seamless experience,' said Que Dallara, Executive Vice President and President of Medtronic Diabetes and CEO Designate of MiniMed. 'We're excited to expand our ecosystem of solutions under one roof with service our customers can count on around the clock.'
This clearance, alongside the previously cleared MiniMed 780G insulin pump as an alternate controller enabled (ACE) pump, completes the Medtronic FDA pre-market approval pathway for the Instinct sensor integration with our MiniMed 780G system for people living with type 1 diabetes. The Instinct sensor, designed exclusively by Abbott for MiniMed automated insulin delivery (AID) and Smart MDI systems, is the world's smallest, thinnest, most discreet integrated CGM (iCGM) and offers a wear time of up to 15 days.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News