Châtillon, France, September 5, 2025
DBV Technologies Establishes an At-The-Market (ATM) Program on Nasdaq
DBV Technologies (Euronext: DBV - ISIN: FR0010417345 - Nasdaq Capital Market: DBVT) (the "Company"), a clinical-stage biopharmaceutical company, today announced that it has filed a prospectus supplement with the U.S. Securities and Exchange Commission ("SEC") relating to an at-the-market offering (the "ATM Program"). Pursuant to this new financing program, the Company may offer and sell, including with unsolicited investors who have expressed an interest, a total gross amount of up to $150 million of American Depositary Shares ("ADS"), each ADS representing five ordinary shares of the Company, from time to time in sales deemed to be an "at the market offering" pursuant to the terms of a sales agreement (the "Sales Agreement") with Citizens JMP Securities, LLC ("Citizens"), acting as sales agent, subject to French regulatory limits. The timing of any sales will depend on a variety of factors. The ATM Program is presently intended to be effective unless terminated in accordance with the Sales Agreement or if ADSs representing the maximum gross amount have been sold thereunder. In connection with the establishment of the ATM Program, the Company has terminated the sales agreement dated as of May 2, 2022, relating to the at-the-market program previously implemented by the Company (the "Prior ATM Program"). The terms and conditions of the ATM Program remain similar to the Prior ATM Program.
The Company currently intends to use the net proceeds (after deduction of fees and expenses related to the financing), if any, of sales of ADSs issued under the program, together with its existing cash and cash equivalents, primarily for activities associated with the Biologics License Application ("BLA"), potential approval and launch of VIASKIN® Peanut patch in toddlers aged 1-3 years old, as well as to advance the development of the Company's product candidates using its proprietary technology platform, VIASKIN, and for working capital and other general corporate purposes, at the Company's discretion.
Pursuant to the Sales Agreement, Citizens, as sales agent, will use commercially reasonable efforts to arrange on the Company's behalf for the sale of all ADSs requested to be sold by the Company to eligible investors requesting it, consistent with Citizens' normal sales and trading practices. Sales prices may vary based on market prices and other factors. Only eligible investors (as described in greater detail below) may purchase ADSs under the ATM Program. In any case, the corresponding sales price of the new ordinary shares underlying the ADSs will not be less than (i) the last closing price of the Company's shares on the regulated market of Euronext in Paris ("Euronext Paris") preceding the setting of the issue price or (ii) the volume-weighted average of the Company's share price on Euronext Paris over a chosen period of between one and five consecutive trading sessions from among the last thirty trading sessions, preceding the setting of the issue price, in each case subject to a maximum discount of 15%.
The ADSs and the underlying ordinary shares will be issued, if any, through one or more share capital increases without shareholders' preferential subscription rights under the provisions of Article L. 225-138 of the French Commercial Code (Code de commerce) and pursuant to the 25th resolution adopted at the Annual General Meeting of Shareholders held on June 11, 2025 (the "2025 Annual General Meeting") (or any substitute resolutions, adopted from time to time), within the limit of a maximum number of 136,948,870 ordinary shares (being the maximum authorized by the shareholders for such resolution), representing a maximum potential dilution of approximately 50% based on the existing share capital of the Company, it being specified that the number of underlying ordinary shares to be admitted on the regulated market of Euronext Paris shall represent, over a rolling period of 12 months, less than 30% of the ordinary shares already admitted to trading on said market without a French listing prospectus or an exemption document.
The new ordinary shares to be sold in the form of ADSs would be issued in one or more offerings at market prices of the ADSs at the time of pricing of the considered capital increase.
Purchases of ADSs under the ATM Program are limited to the categories of investors defined in the 25th resolution adopted at the 2025 Annual General Meeting (or any substitute resolution adopted from time to time): (i) natural person(s) or legal entity(ies), including companies, trusts, investment funds or other investment vehicle(s), regardless of their form, under French or foreign law, investing on a regular basis in the pharmaceutical, biotechnological or medical technology sector, and/or (ii) French or foreign companies, institutions or entities of any form, carrying out a significant portion of their business in the pharmaceutical or chemical sector or in the field of medical devices or research in these areas. The new ordinary shares will be admitted to trading on the regulated market of Euronext in Paris and the issued ADSs will trade on the Nasdaq Capital Market ("Nasdaq").
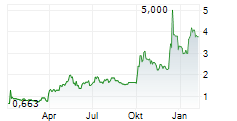
On an illustrative basis, assuming the issuance of the full amount of $150 million (or €128.8 million (all convenience translations in this press release are based on the Euro foreign exchange reference rates of the European Central Bank in effect as of September 2, 2025, of €1.00 = $1.1646) of ADSs under the ATM Program at an assumed offering price of $9.18 (or €7.88), the last reported sale price of the ADSs on Nasdaq on September 2, 2025, a holder of 1.0% of the outstanding Company's share capital as of the date of this press release, would hold 0.63% of the outstanding Company's share capital after the completion of the transaction (calculated on the basis of the number of outstanding shares on the date of publication of this press release), it being specified that, in any event, the number of underlying ordinary shares shall not exceed the limit set forth in the 25th and 31st resolutions adopted by the 2025 Annual General Meeting (or any substitute resolutions adopted from time to time) and shall represent, over a rolling period of 12 months, less than 30% of the ordinary shares already admitted to trading on said market without a French listing prospectus or an exemption document.
During the term of the ATM Program, the Company will include in the publication of its quarterly results information about its use of the ATM Program during the preceding quarter and will also provide an update after each capital increase on a dedicated location on its corporate website in order to inform investors about the main features of each issue that may be completed under the ATM Program from time to time.
A shelf registration statement on Form S-3. Alternatively, a copy of the prospectus supplement (and accompanying prospectus) relating to the offering may be obtained from Citizens JMP Securities, LLC, 450 Park Avenue, 6th Floor, New York, NY 10022, by telephone at (212) 906-3500, or by email at dl-jmp-syndicate@citizensbank.com. No prospectus will be subject to the approval of the French Financial Markets Authority (the Autorité des Marchés Financiers or the "AMF") pursuant to Regulation (EU) 2017/1129 of the European Parliament and of the Council dated June 14, 2017, as amended (the "Prospectus Regulation") since the contemplated share capital increase(s) (for the issuance of the ordinary shares underlying the ADSs) would be offered to qualified investors (as such term is defined in Article 2(e) of the Prospectus Regulation) and fall under the exemption provided for in Article 1(5)(a) of the Prospectus Regulation which states that the obligation to publish a prospectus shall not apply to admission to trading on a regulated market of securities fungible with securities already admitted to trading on the same regulated market, provided that they represent, over a rolling period of 12 months, less than 30% of the number of securities already admitted to trading on the same regulated market.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. In particular, no public offering of the ADSs will be made in Europe.
Information available to the public
Detailed information concerning the Company, in particular with regard to its business, results, forecasts and corresponding risk factors, is provided in the Company's Annual Report on Form 10-K, as amended). The URD as well as other regulated information are available on the AMF website). All of the foregoing documents are available on the Company's website and are available free of charge on request at the Company's registered office at 107 Avenue de la République, 92320 Châtillon, France.
About DBV Technologies
DBV Technologies is a clinical-stage biopharmaceutical company developing treatment options for food allergies and other immunologic conditions with significant unmet medical need. DBV Technologies is currently focused on investigating the use of its proprietary VIASKIN® patch technology to address food allergies, which are caused by a hypersensitive immune reaction and characterized by a range of symptoms varying in severity from mild to life-threatening anaphylaxis. Millions of people live with food allergies, including young children. Through epicutaneous immunotherapy (EPIT), the VIASKIN® patch is designed to introduce microgram amounts of a biologically active compound to the immune system through intact skin. EPIT is a new class of non-invasive treatment that seeks to modify an individual's underlying allergy by re-educating the immune system to become desensitized to allergen by leveraging the skin's immune tolerizing properties. DBV Technologies is committed to transforming the care of food allergic people. The Company's food allergy programs include ongoing clinical trials of VIASKIN Peanut in peanut allergic toddlers (1 through 3 years of age) and children (4 through 7 years of age).
DBV Technologies is headquartered in Châtillon, France, with North American operations in Warren, NJ. The Company's ordinary shares are traded on segment B of Euronext Paris (DBV, ISIN code: FR0010417345) and the Company's ADSs (each representing five ordinary shares) are traded on the Nasdaq Capital Market (DBVT - CUSIP: 23306J309).
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding DBV Technologies' proposed securities offering and its intended use of proceeds. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. These forward-looking statements may be impacted by market conditions as well as other risks and uncertainties set forth in DBV Technologies' regulatory filings with the AMF, DBV Technologies' filings and reports with the SEC, including in DBV Technologies' Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on April 11, 2025 and DBV Technologies' Quarterly Reports on Form 10-Q for the quarters ended March 31, 2025 and June 30, 2025, filed with the SEC on April 30, 2025 and July 29, 2025, respectively, and future filings and reports made with the AMF and SEC by DBV Technologies. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements and estimates, which speak only as of the date hereof. Other than as required by applicable law, DBV Technologies undertakes no obligation to update or revise the information contained in this Press Release.
VIASKIN is a registered trademark of DBV Technologies.
Investor Contact
Katie Matthews
DBV Technologies
katie.matthews@dbv-technologies.com
Media Contact
Brett Whelan
DBV Technologies
brett.whelan@dbv-technologies.com
Disclaimer
This announcement does not, and shall not, in any circumstances constitute a public offering nor an invitation to solicit the interest of the public in France, the United States, or in any other jurisdiction, in connection with any offer.
The distribution of this document may, in certain jurisdictions, be restricted by local legislations. Persons into whose possession this document comes are required to inform themselves about and to observe any such potential local restrictions.
This announcement is not an advertisement and not a prospectus within the meaning of the Prospectus Regulation.
This document does not constitute an offer to the public in France and the securities referred to in this document can only be offered or sold in France to qualified investors (investisseurs qualifiés) as defined in Article 2(e) of the Prospectus Regulation and in accordance with Article L. 411-2 1° of the French Monetary and Financial Code.
With respect to the Member States of the European Economic Area, no action has been undertaken or will be undertaken to make an offer to the public of the securities referred to herein requiring a publication of a prospectus in any relevant Member State. As a result, the securities may not and will not be offered in any relevant Member State except in accordance with the exemptions set forth in Article 1(4) of the Prospectus Regulation or under any other circumstances which do not require the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Regulation and/or to applicable regulations of that relevant Member State.
This document is only being distributed to, and is only directed at, persons in the United Kingdom that (i) are "investment professionals" (people with professional investment experience) falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended, the "Order"), (ii) are persons falling within Article 49(2)(a) to (d) ("high net worth companies, unincorporated associations, etc.") of the Order, or (iii) are persons to whom an invitation or inducement to engage in investment activity (within the meaning of Article 21 of the Financial Services and Markets Act 2000) in connection with the issue or sale of any securities may otherwise lawfully be communicated or caused to be communicated (all such persons together being referred to as "Relevant Persons"). This document is directed only at Relevant Persons and must not be acted on or relied on by persons who are not Relevant Persons. Any investment or investment activity to which this document relates is available only to Relevant Persons and will be engaged in only with Relevant Persons.
Attachment
- PDF Version (https://ml-eu.globenewswire.com/Resource/Download/a36d06b9-f920-4f1e-aff4-5ded7b39ba88)