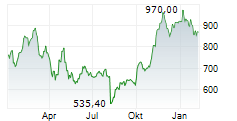
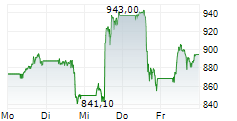
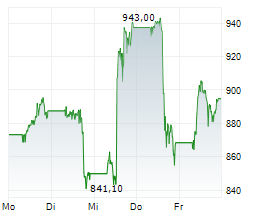
INDIANAPOLIS (dpa-AFX) - Eli Lilly and Co. (LLY) announced Monday positive topline results from the Phase 3 BRUIN CLL-313 clinical trial of Jaypirca (pirtobrutinib), a non-covalent (reversible) Bruton tyrosine kinase (BTK) inhibitor, versus chemoimmunotherapy (bendamustine plus rituximab), in treatment-naïve patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) without 17p deletions.
The study met its primary endpoint, demonstrating a highly statistically significant and clinically meaningful improvement in progression-free survival (PFS) compared to chemoimmunotherapy, as assessed by an independent review committee (IRC), indicating one of the most compelling effect sizes ever observed for a single agent BTK inhibitor in a front-line CLL study.
Overall survival (OS), a key secondary endpoint, was not yet mature at this analysis, but was trending strongly in favor of pirtobrutinib and will be tested for statistical significance at the time of the primary OS analysis, which is anticipated to occur in 2026.
The overall safety profile of pirtobrutinib in BRUIN CLL-313 was generally consistent with previously reported trials across treatment settings.
These data build on the previously reported positive results from the BRUIN Phase 1/2 trial, the Phase 3 BRUIN CLL-321 trial, the first randomized, controlled study ever conducted in an exclusively post-covalent BTK inhibitor population, and the Phase 3 BRUIN CLL-314 trial, the first-ever head-to-head Phase 3 trial versus ibrutinib in CLL to include treatment-naïve patients.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News