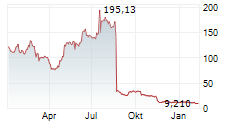
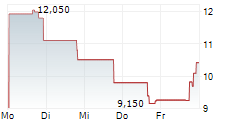
IRVINE, CA / ACCESS Newswire / September 15, 2025 / enVVeno Medical Corporation (Nasdaq:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease, today announced that it will file a request for supervisory appeal of the not-approvable letter from the Center for Devices and Radiological Health (CDRH) of the U.S. Food & Drug Administration (FDA) received on August 19, 2025, in response to its Premarket Approval (PMA) application for the VenoValve®, a surgical replacement venous valve for treating severe deep chronic venous insufficiency (CVI).

The FDA provides several internal informal and formal mechanisms to challenge lower review staff decisions, including scientific controversies. One mechanism is a request for supervisory review in which an appeal is made to the next line of supervision. Supervisory appeals are required to be filed within 30 days of the decision being appealed, which is on or before September 18, 2025. These appeals involve a formal substantive request, an in-person meeting, and a decision. It also often includes multiple interactions even after an initial appeal decision is made.
"Due to our interaction with FDA to obtain our Breakthrough Device Designation (BDD), in which the FDA determined that the VenoValve will meet an unmet clinical need, and our clinical trial negotiations to obtain our Investigational Device Exemption (IDE), as well as during our PMA submission interactions, we have established a productive and collaborative working relationship with the FDA over the past several years. We view this supervisory appeal as an opportunity to extend that relationship," said Robert Berman, enVVeno Medical's Chief Executive Officer. "Bringing a true first-in-class device through the PMA regulatory process raises unique challenges, and it is not unusual to have sequential collaborative discussions with the Agency to address issues that arise during the review process. We are committed to our continuing interactions with the FDA and to the goal of bringing the VenoValve to the 2.5 to 3.5 million patients suffering from severe deep venous CVI in the U.S. and who have no effective treatment options."
Internal Agency reviews are based on information already in the administrative file. Due to the variety of both physician reported and patient reported data generated by the VenoValve pivotal study and which is already a part of the file, the Company is confident that explaining this data to supervisory management in a focused appeal setting will lead to a positive outcome, with a decision expected by the end of 2025.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ: NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of deep venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of severe deep Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The Company is currently performing the final testing necessary to seek IDE approval from the FDA to begin the U.S. pivotal trial for enVVe.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including, but not limited to that such FDA appeal is unsuccessful and other risks detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
NVNO@jtcir.com
(908) 824-0775
MEDIA CONTACT:
Glenn Silver, FINN Partners
Glenn.Silver@finnpartners.com
(973) 818-8198
SOURCE: enVVeno Medical Corporation
View the original press release on ACCESS Newswire:
https://www.accessnewswire.com/newsroom/en/biotechnology/envveno-medical-updates-regulatory-status-of-venovalver-1073026