| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,000 | 0,000 | 18.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 04.02. | enVVeno Medical regains Nasdaq compliance, reports $28 million cash | 3 | Investing.com | ||
| 04.02. | enVVeno Medical Corporation: enVVeno Medical Regains Compliance with Nasdaq Minimum Bid Price Requirement | 271 | ACCESS Newswire | Company achieves minimum $1.00 closing bid for 10 consecutive business days; Nasdaq confirms matter is closedReports Cash and Investments of approximately $28 million as of the Year Ended December 31... ► Artikel lesen | |
| 04.02. | enVVeno Medical Corp - 8-K, Current Report | 2 | SEC Filings | ||
| ENVVENO MEDICAL Aktie jetzt für 0€ handeln | |||||
| 20.01. | enVVeno Medical Corp - 8-K, Current Report | 3 | SEC Filings | ||
| 20.01. | XFRA 5HJ0: AUSSETZUNG/SUSPENSION | 233 | Xetra Newsboard | DAS/ DIE FOLGENDE(N) INSTRUMENT(E) IST/ SIND AB SOFORT AUSGESETZT:THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTILENVVENO MED CORP.NEW... ► Artikel lesen | |
| 20.01. | XFRA NEW INSTRUMENTS AVAILABLE ON 20.01.2026 | 432 | Xetra Newsboard | The following instruments on XETRA do have their first trading 20.01.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 20.01.2026
Aktien
1 DK0010230630 Grønlandsbanken A/S
2... ► Artikel lesen | |
| 19.01. | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 19.01.2026 | 836 | Xetra Newsboard | The following instruments on Boerse Frankfurt do have their last trading day on 19.01.2026Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 19.01.2026ISIN NameBE0003836534 OPTION... ► Artikel lesen | |
| 19.01. | XFRA ISIN CHANGE | 414 | Xetra Newsboard | Einstellung Aufnahme ISIN Name Einstellung mit Ablauf: ISIN Name Ab dem: AnmerkungenBE0003836534 Option N.V. 19.01.2026 BE0974496284 Option N.V. 20.01.2026 Tausch 1000:1US29415J1060 enVVeno Medical... ► Artikel lesen | |
| 19.01. | XFRA 5HJ: AUSSETZUNG/SUSPENSION | 230 | Xetra Newsboard | DAS/ DIE FOLGENDE(N) INSTRUMENT(E) IST/ SIND AB SOFORT AUSGESETZT:THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTILENVVENO MEDICAL... ► Artikel lesen | |
| 19.01. | XFRA CAPITAL ADJUSTMENT INFORMATION - 19.01.2026 | 339 | Xetra Newsboard | Das Instrument TY2B FI4000369947 CITYCON OYJ EQUITY wird ex Kapitalmassnahme gehandelt am 19.01.2026 The instrument TY2B FI4000369947 CITYCON OYJ EQUITY is traded ex capital adjustment on 19.01.2026Das... ► Artikel lesen | |
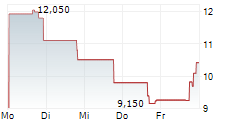
| 15.01. | enVVeno Medical announces reverse stock split; shares down 20% | 1 | Seeking Alpha | ||
| 15.01. | enVVeno Medical Corporation: enVVeno Medical Announces Reverse Stock Split | 307 | ACCESS Newswire | IRVINE, CA / ACCESS Newswire / January 15, 2026 / enVVeno Medical Corporation (NASDAQ:NVNO) ("enVVeno Medical" or the "Company"), today announced that the Company's Board of Directors has approved a... ► Artikel lesen | |
| 12.12.25 | enVVeno Medical Corp - 8-K, Current Report | 2 | SEC Filings | ||
| 26.11.25 | enVVeno Medical Corporation: enVVeno Medical to Participate in a Live Virtual Investor CEO Connect Segment | 312 | ACCESS Newswire | Moderated webcast with Robert Berman, Chief Executive Officer of enVVeno Medical on Wednesday, December 3rd at 4:00 PM ET IRVINE, CA / ACCESS Newswire / November 26, 2025 / enVVeno Medical Corporation... ► Artikel lesen | |
| 20.11.25 | enVVeno Medical senkt Quorum für Hauptversammlungen | 2 | Investing.com Deutsch | ||
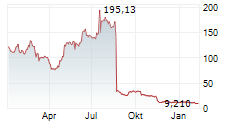
| 14.11.25 | enVVeno Medical: Aktie stürzt nach endgültiger FDA-Absage für VenoValve ab | 10 | Investing.com Deutsch | ||
| 14.11.25 | enVVeno Medical Corp - 8-K, Current Report | - | SEC Filings | ||
| 13.11.25 | FDA rejects enVVeno Medical's appeal for VenoValve approval | 4 | Investing.com | ||
| 13.11.25 | enVVeno Medical Corporation: enVVeno Receives Unfavorable Appeal Decision from the FDA for the VenoValve | 431 | ACCESS Newswire | IRVINE, CA / ACCESS Newswire / November 13, 2025 / enVVeno Medical Corporation (NASDAQ:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease... ► Artikel lesen | |
| 31.10.25 | enVVeno Medical reports Q3 results | 3 | Seeking Alpha |
1 2 3 Weiter >>
43 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS MEDICAL CARE | 39,450 | +0,59 % | GOLDMAN SACHS stuft FMC FRESENIUS MEDICAL CARE AG auf 'Neutral' | NEW YORK (dpa-AFX Analyser) - Die US-Investmentbank Goldman Sachs hat die Einstufung für FMC mit einem Kursziel von 40 Euro auf "Neutral" belassen. Das Ende des Jahres 2025 sei stark gewesen, schrieb... ► Artikel lesen | |
| SIEMENS HEALTHINEERS | 42,140 | -0,59 % | RBC stuft Siemens Healthineers auf 'Outperform' | NEW YORK (dpa-AFX Analyser) - Die kanadische Bank RBC hat Siemens Healthineers mit einem Kursziel von 55 Euro auf "Outperform" belassen. Die Nervosität der Anleger vor einem möglichen Verkauf des Diagnostikgeschäfts... ► Artikel lesen | |
| CARL ZEISS MEDITEC | 27,120 | -0,59 % | Carl Zeiss Meditec AG: ZEISS launches Collaborative Care application to strengthen continuity of care; new solution offers on-premises and cloud-based options | Built on the ZEISS Health Data Platform, ZEISS Collaborative Care offers eye care professionals a flexible way to collaborate - whether as a standalone cloud application or as an integrated... ► Artikel lesen | |
| ECKERT & ZIEGLER | 15,420 | +1,65 % | EQS-AFR: Eckert & Ziegler SE: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114, 115, 117 WpHG | EQS Vorabbekanntmachung Finanzberichte: Eckert & Ziegler SE
/ Vorabbekanntmachung über die Veröffentlichung von Rechnungslegungsberichten
Eckert & Ziegler SE: Vorabbekanntmachung... ► Artikel lesen | |
| GERRESHEIMER | 16,950 | +7,96 % | SDAX-Wert im Bafin-Fokus: Gerresheimer stürzt immer tiefer | Der Druck auf den Düsseldorfer Verpackungsspezialisten steigt: Die Bundesanstalt für Finanzdienstleistungsaufsicht BaFin hat angekündigt, eine bereits laufende Bilanzkontrolle bei Gerresheimer auszuweiten... ► Artikel lesen | |
| MEDTRONIC | 82,50 | -0,18 % | Medtronic Launches MiniMed Go Smart MDI System In Europe | WASHINGTON (dpa-AFX) - Medtronic (MDT) announced the commercial launch in Europe of the MiniMed Go Smart MDI system with the Simplera sensor. The launch will be rolled out gradually across Europe... ► Artikel lesen | |
| INTUITIVE SURGICAL | 425,20 | -0,18 % | Im Krankenhaus auf Aktiensuche: Paul Hartmann, Technogym und Intuitive Surgical | Ein paar Dinge habe ich nach meinen Krankenhaus-Aufenthalten im November, Dezember und Januar gelernt...
...zum Beispiel: Es ist egal, wie schlecht es dir geht, es geht immer noch jemandem schlechter... ► Artikel lesen | |
| THERMO FISHER | 439,40 | -0,36 % | THERMO FISHER SCIENTIFIC INC. - 10-K, Annual Report | ||
| TELADOC HEALTH | 4,333 | -2,40 % | Teladoc Health Q4 Loss Narrows | WASHINGTON (dpa-AFX) - Teladoc Health, Inc. (TDOC) on Wednesday reported a fourth-quarter net loss of $25.1 million, or $0.14 per share, compared to $48.4 million or $0.28 per share last year.Fourth-quarter... ► Artikel lesen | |
| STRATEC | 21,100 | +0,24 % | EQS-PVR: STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: STRATEC SE
STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide... ► Artikel lesen | |
| RHOEN-KLINIKUM | 13,100 | +1,55 % | EQS-PVR: RHÖN-KLINIKUM AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung | EQS Stimmrechtsmitteilung: RHÖN-KLINIKUM Aktiengesellschaft
RHÖN-KLINIKUM AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung
20.02.2026... ► Artikel lesen | |
| ROKU | 82,43 | -1,01 % | Roku Swings To Q4 Profit | WASHINGTON (dpa-AFX) - Roku, Inc. (ROKU) on Thursday reported fourth-quarter net income of $80.5 million or $0.53 per share, compared to a net loss of $35.5 million or $0.24 per share last year.Revenues... ► Artikel lesen | |
| EUROFINS SCIENTIFIC | 67,64 | -0,32 % | Eurofins Scientific SE: Eurofins announces the publication of its 2025 Annual Report and 2025 Environmental, Social and Governance Report | Regulatory News:
Eurofins (Paris:ERF) has filed its 2025 Annual Report and its 2025 Environmental, Social and Governance Report for the year ended 31 December 2025 with the electronic database... ► Artikel lesen | |
| FRESENIUS | 50,90 | -0,82 % | DAX-Check: BMW, Fresenius, Hochtief, MTU, Vonovia, Zalando | Donald Trump wirbelt die Märkte mal wieder durcheinander. Der US-Präsident kündigte neue globale Zölle an. Zuvor hatte der Supreme Court die bisherigen Zollmaßnahmen für unrechtmäßig erklärt. Die Unsicherheit... ► Artikel lesen | |
| ALIGNMENT HEALTHCARE | 19,220 | -5,83 % | Alignment Healthcare Reports Fourth Quarter and Full-Year 2025 Results; Beats High-End of Guidance Across All Key Metrics | Delivers full-year revenue of $3.95 billion, representing 46.1% growth year-over-year Exceeds high-end of fourth quarter and full-year guidance across all key metrics: membership, revenue, adjusted... ► Artikel lesen |
ENVVENO MEDICAL CORPORATION gehört der Branche Gesundheitswesen an. Die relative Kursveränderung beträgt aktuell +1,55 % bei einem Aktienkurs von 10,510 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ENVVENO MEDICAL CORPORATION finden Sie auf Wallstreet Online.