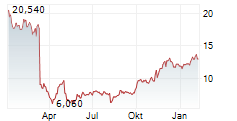
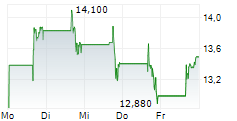
NEW YORK CITY (dpa-AFX) - Arvinas Inc. (ARVN) and Pfizer Inc. (PFE) announced plans to jointly select a third party for the out-licensing and commercialization of vepdegestrant. As part of its operational streamlining, Arvinas will reduce its workforce by an additional 15%, with the most significant cuts affecting roles tied to vepdegestrant commercialization. In a parallel move, the Arvinas Board of Directors has authorized a stock repurchase program of up to $100 million of the company's common shares.Thecompany reaffirmed its cash runway guidance into the second half of 2028.
Arvinas and Pfizer agreed to out-license the commercialization rights to vepdegestrant to a third party. Together, the companies have begun seeking a partner with the capabilities and expertise to maximize the commercial potential of vepdegestrant, if approved, for patients with ESR1-mutant, ER+/HER2- advanced or metastatic breast cancer and potentially develop vepdegestrant in new settings.
Vepdegestrant is currently under review by the U.S. Food and Drug Administration (FDA) as a monotherapy in the treatment of estrogen receptor positive (ER+), human epidermal growth factor receptor 2negative (HER2-), ESR1-mutated advanced or metastatic breast cancer previously treated with endocrine-based therapy. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) action date of June 5, 2026.
Arvinas said it continues to believe that its pipeline of differentiated PROTAC degraders has the potential to create important therapies for patients with debilitating and life-threatening diseases across oncology and neuroscience. Arvinas currently has three investigational PROTAC degraders in Phase 1 trials: ARV-102, a LRRK2 degrader for progressive supranuclear palsy and Parkinsons disease; ARV-393, a BCL6 degrader for subsets of non-Hodgkin lymphoma; and ARV-806, a KRAS G12D degrader for solid tumor malignancies.
Arvinas has announced further changes to its organizational and cost structures following a shift in its development strategy for the vepdegestrant program and a renewed focus on early-stage pipeline programs.
As part of this effort, Arvinas will limit additional spending on the vepdegestrant program, focusing only on activities necessary for commercialization readiness and the identification of a third-party partner for out-licensing, in coordination with Pfizer. The company will also reduce its workforce by an additional 15%, with the most significant cuts affecting roles tied to vepdegestrant commercialization.
In addition, Arvinas plans to proactively manage pipeline costs by pursuing strategic business development opportunities and identifying further operational efficiencies across the organization. These steps build on previous actions taken by the Board and management to strengthen the company's financial position and improve overall efficiency.
Together with the approximately $80 million in annual cost savings announced on May 1, 2025, the planned out-licensing of vepdegestrant and related cost-cutting measures are expected to generate total annual savings of more than $100 million compared to fiscal year 2024.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News