WASHINGTON (dpa-AFX) - Medtronic plc (MDT), Friday announced that its Altaviva device, designed to treat urge urinary incontinence, has secured U.S. Food and Drug Administration approval.
Designed for real-life needs, the Altaviva device is MRI-compatible, providing patients with peace of mind for planned or unexpected medical imaging, the company stated.
The device is inserted near the ankle during a minimally invasive procedure. It sits slightly below the skin and sends electrical impulses to the tibial nerve, helping restore communication between the bladder and the brain to regulate bladder control.
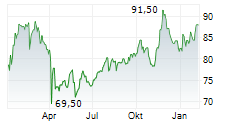
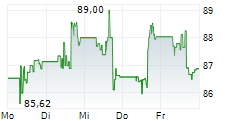
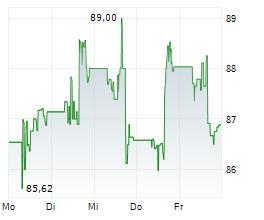
In the pre-market hours, MDT is trading at $96.05, up 0.49 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News