PARIS (dpa-AFX) - Ipsen (IPSEY), Friday announced that Japan's Ministry of Health, Labour and Welfare has approved Bylvay also known as odevixibat for the treatment of pruritus associated with progressive familial intrahepatic cholestasis, a rare genetic liver disorder that can lead to progressive liver damage and liver failure.
PFIC causes debilitating symptoms, most notably severe itching, which disrupts sleep, impacts cognitive and social development, and severely diminishes quality of life.
Bylvay is a once-daily oral ileal bile acid transport inhibitor that reduces bile acid reabsorption into the liver. In the global Phase III PEDFIC trial, children treated with Bylvay showed significant improvements in bile acid levels and pruritus severity, with treatment generally well-tolerated and a low incidence of gastrointestinal events.
The Japanese approval was supported by a Phase III open-label study in pediatric patients with PFIC types 1 and 2, confirming results consistent with the PEDFIC trial. The clinical program in Japan was managed by Jadeite Medicines, with Ipsen now responsible for commercialization.
Bylvay has already been approved in the EU and US as the first treatment for PFIC and has since received additional approvals for cholestatic pruritus in Alagille syndrome. The therapy is also in late-stage development for biliary atresia under the Phase III BOLD trial.
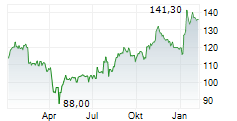
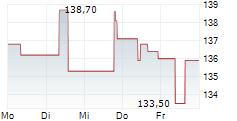
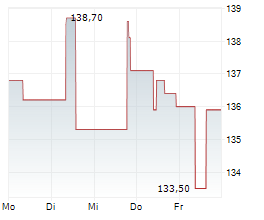
IPSEY is currently trading at $33.87, down $0.42 or 1.22 percent on the OTC Market.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News