| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
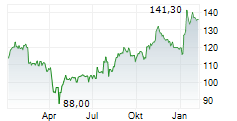
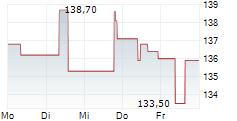
| 162,80 | 163,90 | 28.02. | |
| 163,90 | 164,80 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Ipsen Pharma: Ipsen receives positive CHMP opinion for Ojemda for the treatment as monotherapy of children with relapsed or refractory BRAF-altered pediatric low-grade glioma | 344 | GlobeNewswire (Europe) | If approved, Ojemda (tovorafenib) is expected to be the first and only targeted medicine in European Union for children with relapsed or refractory BRAF-altered pediatric low-grade glioma, irrespective... ► Artikel lesen | |
| Fr | Quotient and Ipsen extend partnership for ultra-rare disease therapy | 2 | Pharmaceutical Technology | ||
| 19.02. | Ipsen Pharma: Ipsen S.A. publishes its 2025 Consolidated Financial Statements | 1 | GlobeNewswire (USA) | ||
| IPSEN Aktie jetzt für 0€ handeln | |||||
| 12.02. | Genfit adds $20M as Ipsen records strong sales for liver drug | 6 | Seeking Alpha | ||
| 12.02. | GENFIT S.A.: GENFIT to receive US$20M milestone after Ipsen's Iqirvo exceeds the US$200M threshold in its first full year of net sales | 223 | GlobeNewswire (Europe) | Ipsen reported US$88M of Iqirvo® net sales in PBC for 4Q25, bringing 2025 sales to US$208M, triggering the first commercial milestone payment to GENFIT ahead of schedule1Ipsen confirmed its commitment... ► Artikel lesen | |
| 12.02. | Ipsen GJ 2025: Zweistelliges Wachstum beflügelt ambitionierten Ausblick für 2026 | 2 | Investing.com Deutsch | ||
| 12.02. | Ipsen übertrifft Prognosen in Q4 2025 - Aktie legt deutlich zu | 6 | Investing.com Deutsch | ||
| 12.02. | Ipsen Full-Year Profit Rises On Higher Sales; Sees Over 13% Sales Growth In 2026 | 360 | AFX News | PARIS (dpa-AFX) - Ipsen S.A. (IPSEY), a specialty-care biopharmaceutical company, on Thursday reported higher earnings for 2025, supported by stronger sales performance.Net income attributable... ► Artikel lesen | |
| 12.02. | Ipsen S.A. Non-GAAP EPS of €12.09, revenue of €3.68B; sets full-year 2026 guidance and mid-term outlook | 6 | Seeking Alpha | ||
| 12.02. | Ipsen Pharma: Ipsen delivers strong results in 2025, driven by solid execution across all therapeutic areas, and provides 2026 guidance | 641 | GlobeNewswire (Europe) | FY 2025 sales growth of 10.9% at CER1, or 8.1% as reported, driven by growth of the three therapeutic areas of Oncology (4.1%1), Rare Disease (102.5%1) and Neuroscience (9.7%1); Somatuline sales growth... ► Artikel lesen | |
| 09.02. | Ipsen Pharma: Ipsen - January 2026 - Monthly information relative to the total number of voting rights and shares composing the share capital | 1 | GlobeNewswire (USA) | ||
| 29.01. | Ipsen Pharma: Ipsen nominates Peter Guenter to its Board of Directors | 438 | GlobeNewswire (Europe) | PARIS, FRANCE, 29 January 2026 - Ipsen (Euronext: IPN; ADR: IPSEY) announced today the co-optation of Peter Guenter to its Board as a Director, effective January 28, 2026, further to the vacancy of... ► Artikel lesen | |
| 28.01. | Ipsen appoints Pierrick Lefranc as Executive Vice President Technical Operations | 3 | PMLiVE | ||
| 27.01. | Ipsen Pharma: Pierrick Lefranc appointed as Executive Vice President Technical Operations, member of Executive Leadership Team | 455 | GlobeNewswire (Europe) | PARIS, FRANCE, 27 January 2026 - Ipsen (Euronext: IPN; ADR: IPSEY) announced today that Pierrick Lefranc will be appointed as Executive Vice President (EVP) and member of the Executive Leadership Team... ► Artikel lesen | |
| 22.01. | Galderma und Ipsen beenden F&E-Partnerschaft für Neuromodulatoren nach Schiedsspruch | 20 | Investing.com Deutsch | ||
| 21.01. | Ipsen Pharma: Arbitration tribunal upholds Ipsen's termination of R&D agreement with Galderma | 475 | GlobeNewswire (Europe) | PARIS, FRANCE, 21 January 2026 - Ipsen (Euronext: IPN; ADR: IPSEY) announced that the Arbitral Tribunal of the International Chamber of Commerce (ICC) has issued a final decision in favor of Ipsen,... ► Artikel lesen | |
| 14.01. | Ipsen rises on FDA breakthrough therapy status for blood cancer therapy | 3 | Seeking Alpha | ||
| 14.01. | Ipsen Pharma: New data reinforces Ipsen's commitment to bringing solutions and addressing care gaps in neurological diseases at TOXINS | 788 | GlobeNewswire (Europe) | 14 abstracts will be presented across a range of neurological conditions including post-stroke spasticity, cervical dystonia, blepharospasm and other movement disordersInterim data from the ongoing... ► Artikel lesen | |
| 14.01. | FDA Grants Breakthrough Therapy Designation To Ipsen's IPN60340 In Acute Myeloid Leukemia | 511 | AFX News | PARIS (dpa-AFX) - Ipsen (IPSEY, IPN.PA) announced that the U.S. Food and Drug Administration has granted Breakthrough Therapy Designation for IPN60340 in combination with venetoclax and azacitidine... ► Artikel lesen | |
| 13.01. | Ipsen Pharma: U.S. FDA grants Ipsen's IPN60340 (ICT01) Breakthrough Therapy Designation in first line unfit Acute Myeloid Leukemia | 571 | GlobeNewswire (Europe) | Breakthrough Therapy Designation granted for investigational therapy IPN60340 in combination with venetoclax and azacitidine in first line unfit acute myeloid leukemia
PARIS, FRANCE, 13 JANUARY... ► Artikel lesen |
1 2 3 4 5 Weiter >>
105 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 42,010 | +0,39 % | Bayer Aktie: Kursrally am absoluten Limit - droht jetzt der große Rücksetzer? | © Foto: sbDie Bayer-Aktie hat in kurzer Zeit eine beeindruckende Aufholjagd hingelegt. Vom Tief bei unter 20 Euro schoss der Kurs auf knapp 50 Euro - mehr als eine Verdopplung. Klingt gut, aber genau... ► Artikel lesen | |
| NOVO NORDISK | 31,600 | -0,24 % | Rückschlag im Abnehm-Boom: Lilly schlägt Novo deutlich | Der globale Markt für Abnehmmedikamente wächst rasant - doch der Wettbewerb verschärft sich. Neue Studiendaten setzen einen der führenden Anbieter spürbar unter Druck. Für Anleger rückt damit die Frage... ► Artikel lesen | |
| PFIZER | 23,350 | -0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,44 | +0,21 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 127,75 | +1,67 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 125,66 | -0,33 % | Gilead Sciences auf Einkaufstour: Wer folgt als Nächstes? | Gilead Sciences lässt seinen Worten Taten folgen: Mit einer ersten Multi-Milliarden-Übernahme im laufenden Jahr stärkt der Pharmakonzern seine Pipeline. Weiterhin ausstehend: eine bedeutende Transaktion... ► Artikel lesen | |
| AURORA CANNABIS | 3,240 | -1,22 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,41 | -0,40 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,040 | +0,93 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,00 | -0,31 % | Rekordquartal und AbbVie-Deal beflügeln Gubra-Aktie: Kursplus von 13,88 % | ||
| ROCHE | 402,50 | -0,27 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,950 | +0,85 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 889,90 | -0,11 % | Novo posts worst monthly decline as Lilly extends lead | ||
| MERCK & CO | 104,60 | -0,19 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen |
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu IPSEN SA finden Sie auf Wallstreet Online.