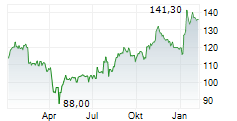
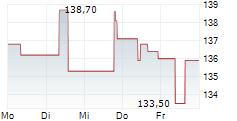
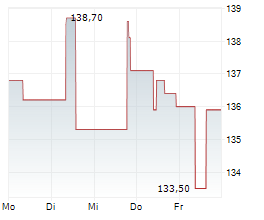
PARIS (dpa-AFX) - Ipsen (IPSEY) said on Friday that its pivotal Phase II FALKON study evaluating fidrisertib in patients with fibrodysplasia ossificans progressiva (FOP) failed to meet its primary endpoint, prompting the company to close the trial.
The primary endpoint was the reduction of new heterotopic ossification, or abnormal bone formation in soft tissues, in patients with FOP, an extremely rare genetic disorder in which abnormal bone formation progressively replaces muscles and connective tissues.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News