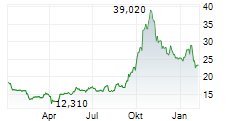
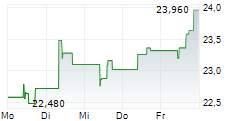
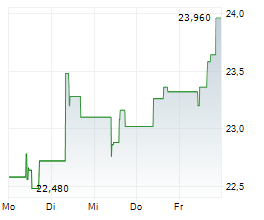
PETAH TIKVA (dpa-AFX) - Medincell SA (MEDCL.PA) reported that new long-term safety data from the completed Phase 3 SOLARIS trial support the potential of Olanzapine LAI (TEV-749) as the first long-acting olanzapine treatment option for schizophrenia, with no post-injection delirium/sedation syndrome (PDSS) observed. The data were presented by Teva Pharmaceuticals.
Olanzapine LAI (mdc-TJK / TEV-749) is an investigational, once-monthly, subcutaneous long-acting injection of the atypical antipsychotic olanzapine. It is the second product developed under the Teva.
Teva plans to proceed with an NDA submission of Olanzapine LAI in the US in the fourth quarter of 2025.
Medincell's partner Teva leads the clinical development and regulatory process and is responsible for commercialization of the Olanzapine LAI. Medincell is entitled to receive royalties on net sales, along with development and commercial milestone payments.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News