| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
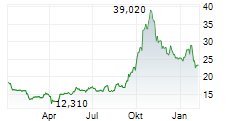
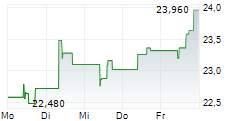
| 22,640 | 23,140 | 13:02 | |
| 23,060 | 23,400 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Medincell to Present at the TD Cowen 46th Annual Healthcare Conference in Boston, March 2-4, 2026 | 229 | Business Wire | Regulatory News:
Medincell (Euronext Paris: MEDCL), a commercial- and clinical-stage biopharmaceutical licensing company developing long-acting injectable treatments (the "Company"), today announced... ► Artikel lesen | |
| 21.02. | Teva, Medincell granted FDA review for long-acting antipsychotic | 16 | Seeking Alpha | ||
| 21.02. | FDA Accepts NDA For Teva And Medincell's Olanzapine LAI In Schizophrenia Treatment | 795 | AFX News | PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, the U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), together with Medincell, announced that the U.S. Food and Drug Administration... ► Artikel lesen | |
| 20.02. | Medincell: U.S. Food and Drug Administration (FDA) Accepts Teva's New Drug Application (NDA) for Olanzapine Extended-Release Injectable Suspension (TEV-'749) for the Once-Monthly Treatment of Schizophrenia in Adults | 451 | Business Wire | Olanzapine long-acting injectable suspension (TEV-'749) has the potential to offer the efficacy of olanzapine in a once-monthly, subcutaneous formulation 1 If approved, TEV-'749 could help... ► Artikel lesen | |
| MEDINCELL Aktie jetzt für 0€ handeln | |||||
| 10.02. | Medincell's ISS ESG Corporate Rating Upgraded | 297 | Business Wire | Medincell's overall ESG rating has been upgraded from C+ to B by ISS, a leading global provider of sustainability ratings Medincell is ranked in the first decile of the Pharmaceuticals Biotechnology... ► Artikel lesen | |
| 28.01. | Medincell - UZEDY: Net Sales Increased from $117M in 2024 to $191M in 2025 (+63%) | 357 | Business Wire | OLANZAPINE LAI: EU Submission Expected in Q2 2026
Regulatory News:
Medincell's (Paris:MEDCL) partner, Teva Pharmaceuticals, today shared the following information in connection with the publication... ► Artikel lesen | |
| 20.01. | Medincell: Half-year Liquidity Contract Statement | 280 | Business Wire | Regulatory News:
Under the liquidity contract entrusted by Medincell (Paris:MEDCL) to Rothschild Martin Maurel, the following resources were included in the liquidity account at December 31, 2025:... ► Artikel lesen | |
| 12.01. | Medincell: Publication of the 2026 Financial Calendar | 354 | Business Wire | Regulatory News:
Medincell (Paris:MEDCL):
Event Date
Annual results 2025-2026
(April 2025-March 2026)
Tuesday, June 16, 2026
General Meeting
Thursday... ► Artikel lesen | |
| 09.12.25 | Medincell: Starkes Umsatzwachstum, doch anhaltende Verluste belasten den Aktienkurs | 4 | Investing.com Deutsch | ||
| 09.12.25 | Medincell Publishes its Consolidated Half-Year Financial Results | 451 | Business Wire | (April 1st, 2025 September30, 2025)
Regulatory News:
Christophe Douat, CEO of Medincell (Paris:MEDCL)"We are pleased with the company's growth and momentum. We have entered the most transformative... ► Artikel lesen | |
| 09.12.25 | Medincell's Partner Teva Pharmaceuticals Announces the New Drug Application1 Submission to U.S. FDA for Olanzapine Extended-Release Injectable Suspension (TEV-'749 / mdc-TJK) for the Once-Monthly Treatment of Schizophrenia in Adults | 484 | Business Wire | Regulatory News:
Medincell (Paris:MEDCL):
Olanzapine long-acting injectable (LAI) has the potential to offer the efficacy of olanzapine in a once-monthly, subcutaneous formulation, for a broad... ► Artikel lesen | |
| 01.12.25 | Medincell: Videoconference and Publication of Half-year Financial Results, Tuesday, December 9, 2025 | 396 | Business Wire | Regulatory News:
Medincell (Paris:MEDCL) will hold a videoconference on Tuesday December 9, 2025 to present the half-year financial results (April 2025-September 2025)
Meeting in French, 6:00... ► Artikel lesen | |
| 27.11.25 | Medincell Management to Present at the 8th Annual Evercore Healthcare Conference and at the Piper Sandler 37th Annual Healthcare Conference | 489 | Business Wire | Regulatory News:
Medincell (Paris:MEDCL):
Christophe Douat, Chief Executive Officer, Dr. Richard Malamut, Chief Medical Officer, and Dr Grace Kim, Chief Strategy Officer, U.S. Finance, will... ► Artikel lesen | |
| 24.11.25 | Medincell Awarded New Grant to Fight Malaria | 372 | Business Wire | Medincell to receive a $3 million grant from the Gates Foundation to advance mdc-STM malaria program. mdc-STM program is an investigational, three-month, subcutaneous injectable formulation of... ► Artikel lesen | |
| 13.11.25 | Medincell Management to Participate in Fireside Chat at the Jefferies London Healthcare Conference 2025 (London - Nov. 17-20, 2025) | 394 | Business Wire | Regulatory News:
Christophe Douat, Chief Executive Officer, and Dr. Richard Malamut, Chief Medical Officer, will present Medincell's (Paris:MEDCL) corporate overview during a fireside chat at the... ► Artikel lesen | |
| 11.11.25 | Medincell Promotes Grace Kim Chief Strategy Officer U.S. Finance | 1 | Contract Pharma | ||
| 11.11.25 | Medincell Appoints Dr Grace Kim, Chief Strategy Officer, U.S. Finance, to Advance into Next Stage of US Capital Growth | 412 | Business Wire | Regulatory News:
Medincell (Paris:MEDCL), a clinical-stage pharmaceutical company pioneering long-acting injectable therapies, today announced the expanded role of Dr Grace Kim, Chief Strategy... ► Artikel lesen | |
| 10.11.25 | Medincell to Join MSCI World Small Cap Index, a Leading Global Benchmark | 392 | Business Wire | Medincell has been selected for inclusion in the Morgan Stanley Capital International (MSCI) World Small Cap Index, which encompasses the most liquid and high-performing small-cap companies across... ► Artikel lesen | |
| 06.11.25 | Medincell to Participate in the UBS Global Healthcare Conference (Palm Beach) and the Stifel Healthcare Conference (New-York) | 449 | Business Wire | Regulatory News:
Dr Grace Kim, Chief Strategy Officer, U.S. Finance, and Dr Richard Malamut, Chief Medical Officer, will speak on Medincell's (Paris:MEDCL) value proposition, including recent... ► Artikel lesen | |
| 05.11.25 | Medincell: UZEDY Continues Strong Growth; Teva Setting the Stage for US NDA Submission for Olanzapine LAI in Q4 2025 | 614 | Business Wire | Regulatory News:
Medincell's (Paris:MEDCL) partner Teva Pharmaceuticals shared today the following information:
About Olanzapine Long-Acting Injectable (TEV-749 mdc-TJK)
1-Month subcutaneous... ► Artikel lesen |
1 2 3 Weiter >>
48 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen |
Das Unternehmen MEDINCELL SA kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +0,34 % bei einem Aktienkurs von 23,320 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MEDINCELL SA finden Sie auf Wallstreet Online.