PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, the U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), together with Medincell, announced that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) for olanzapine extended-release injectable suspension (TEV-'749). This investigational therapy is being developed for the treatment of schizophrenia in adults.
Currently, there is no long-acting olanzapine formulation available without an FDA-required Risk Evaluation and Mitigation Strategy (REMS). The REMS program mandates administration in a certified healthcare facility and requires patients to remain under observation for three hours following injection.
The Phase 3 SOLARIS trial evaluated TEV-'749 as a once-monthly subcutaneous injection. Results showed that its efficacy and safety profile was consistent with existing olanzapine formulations, while importantly demonstrating no evidence to support the need for post-injection monitoring.
TEV-'749, developed by Teva Pharmaceuticals and Medincell, represents a potential advancement in schizophrenia treatment. It offers a once-monthly subcutaneous long-acting injectable (LAI) of the second-generation atypical antipsychotic olanzapine. However, it remains investigational and has not yet been approved by any regulatory authority for clinical use.
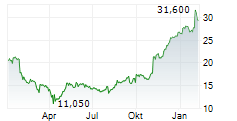
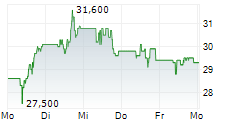
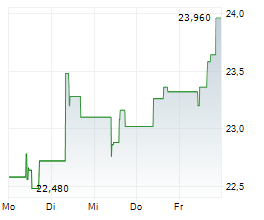
TEVA closed Friday's regular trading at $34.22 down $0.11 or 0.32%. In after-hours trading, the stock slipped further to $33.90, down $0.32 points or 0.94%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News