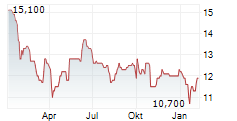
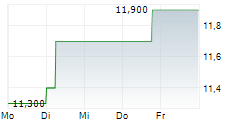
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - Dr. Reddy's Laboratories (DRREDDY, RDY) announced that the European Medicines Agency's Committee for Medicinal Products for Human Use has adopted a positive opinion recommending marketing authorisation for AVT03, a biosimilar of Prolia or denosumab and Xgeva or denosumab in European markets. The company said a Marketing Authorisation Application for submission to the UK Medicines and Healthcare products Regulatory Agency will be made separately.
In May 2024, Dr. Reddy's and Alvotech entered into a license and supply agreement for the commercialization of AVT03. Under the agreement, Alvotech will develop and manufacture AVT03, while Dr. Reddy's is responsible for registration and commercialization in applicable markets, including the U.S. and Europe.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News