- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 13:45 | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 06:02 | Dr Reddy's Lab gets EIR from USFDA for Srikakulam facility | 1 | capitalmarket.com | ||
| 27.02. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 25.02. | Fundamental & Technical Picks: Tata Motors PV, Infosys, Dr Reddy's, RVNL, Biocon, other recommendations | 2 | ZEE Business | ||
| 25.02. | Stock Alert: HG Infra, IRFC, RVNL, Schaeffler India, Dr Reddys Lab, Waaree Energies | 27 | capitalmarket.com | ||
| 20.02. | Dr. Reddy's biosimilar for Bristol's Orencia undergoes FDA review | 1 | Seeking Alpha | ||
| DR REDDYS Aktie jetzt für 0€ handeln | |||||
| 19.02. | Dr. Reddy's Labs acquires 'Progynova' portfolio from UK's Mercury Pharma Group | 1 | capitalmarket.com | ||
| 18.02. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 10.02. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 02.02. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 30.01. | Coya Therapeutics Sets $11.1 Million Private Placement Led by Dr. Reddy's And Greenlight Capital | 2 | pulse2.com | ||
| 30.01. | Coya Therapeutics raises $11.1M in private placement led by Dr. Reddy's | 7 | Seeking Alpha | ||
| 22.01. | Dr. Reddy's Q3 Earnings Match Estimates, Revenues Rise Y/Y | 7 | Zacks | ||
| 22.01. | Gainers & Losers: IIFL Finance, Dr Reddy's among 7 stocks that moved most on Thursday | 2 | Times of India | ||
| 22.01. | Dr. Reddy's Labs ADR: EPS übertrifft Schätzungen um 14,36 $ - Umsatz besser als erwartet | 3 | Investing.com Deutsch | ||
| 22.01. | Dr Reddy's shares surge 5% despite 14% fall in profit? Why brokerages are bullish | 5 | ZEE Business | ||
| 22.01. | Dr. Reddy's GAAP EPS of Rs 14.52, revenue of Rs 87.27B | 3 | Seeking Alpha | ||
| 22.01. | Dr Reddy's Lab Q3 PAT drops 14% YoY to Rs 1,210 cr | 2 | capitalmarket.com | ||
| 22.01. | Stock Alert: Eternal, Bajaj Consumer, Dr Reddys Lab, KEI Industries, PNB Housing | 1 | capitalmarket.com | ||
| 21.01. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 2 | SEC Filings |
1 2 3 4 5 Weiter >>
86 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 36,390 | -3,67 % | Revolution in der Krebstherapie: Vidac Pharma attackiert den Krebs-Thron der Großen! Warum der kleinere Mitbewerber Riesen wie Bayer und BioNTech die Show stehlen könnte! | Die Welt der Biotechnologie und Pharmazie ist aktuell in Aufruhr, denn technologische Durchbrüche und strategische Neuausrichtungen versprechen eine spannende Zukunft für Anleger. Besonders im Fokus... ► Artikel lesen | |
| NOVO NORDISK | 33,290 | -0,76 % | Novo Nordisk: Aktie fällt immer weiter - das ist jetzt wichtig | Die Aktie von Novo Nordisk hat zu Wochenbeginn erneut kräftig nachgegeben, nachdem die Dänen enttäuschende Daten zum Hoffnungsträger CagriSema veröffentlicht haben. Auch ein Deal zur Wochenmitte konnte... ► Artikel lesen | |
| PFIZER | 23,000 | +0,35 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 137,50 | -0,94 % | HV-Termine: Hauptversammlungen bei BRAIN Biotech, LS Telcom, Metro, MVV Energie, Novartis | Einmal jährlich müssen sich Aufsichtsrat und Vorstand einer Gesellschaft den Aktionären stellen: Die Hauptversammlung ist das höchste Organ einer Aktiengesellschaft und vergleichbarer Unternehmens-Formen.... ► Artikel lesen | |
| MERCK KGAA | 110,75 | -2,16 % | Tagesvorschau 5. März: Marktausblick am Donnerstag: DHL, Merck und Aareal Bank öffnen ihre Bücher | Donnerstag: Immobilien, Industrie und WelthandelDer Donnerstag steht ganz im Zeichen großer deutscher Konzerne und wichtiger US-Konjunkturdaten. Mit Zahlen unter anderem von DHL Group, Merck KGaA, Aareal... ► Artikel lesen | |
| GILEAD SCIENCES | 124,06 | -0,80 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,000 | -0,99 % | Im Schatten des Ölpreisschocks: Bereiten Tilray, Aurora Cannabis, Canopy & Co. ein spektakuläres Comeback vor? | © Foto: adobe.stock.comDer Iran-Krieg überschattet derzeit das Handelsgeschehen an den Finanzmärkten. Der rasante Anstieg der Ölpreise überschattet so ziemlich alles - auch ein mögliches Comeback der... ► Artikel lesen | |
| SANOFI | 77,16 | -1,14 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 23,530 | -1,34 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach monatlichen Absatzdaten für Medikamente mit einem Kursziel von 1700 Pence auf "Underweight" belassen. Der Umsatz mit... ► Artikel lesen | |
| ABBVIE | 197,40 | -1,55 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 378,60 | -0,38 % | Roche erzielt klinischen Erfolg mit Multiple-Sklerose-Medikament | DJ Roche erzielt klinischen Erfolg mit Multiple-Sklerose-Medikament
Von Adria Calatayud
DOW JONES--Der Schweizer Pharmakonzern Roche hat mit seinem Medikamentenkandidat gegen Multiple Sklerose... ► Artikel lesen | |
| CANOPY GROWTH | 0,917 | +0,88 % | Is Canopy Growth Stock Going to $0? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 848,10 | +0,14 % | Ausschreibung KONKRET-Preis 2026: Neues Setting, erhöhtes Preisgeld / Lilly Deutschland Stiftung intensiviert Innovationsförderung in der Gesundheitsversorgung | Bad Homburg (ots) - Mit dem Innovationspreis KONKRET fördert die Lilly Deutschland Stiftung Projekte, die Lücken und Barrieren in der Gesundheitsversorgung adressieren. Aufbauend auf bisherigen Erfolgen... ► Artikel lesen | |
| MERCK & CO | 98,60 | -1,40 % | MERCK & CO INC: Schwäche im Fokus, Chance im Detail |
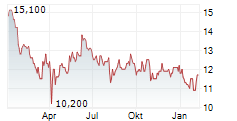
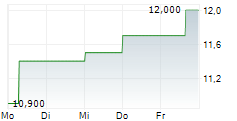
Das Unternehmen DR REDDYS LABORATORIES LTD ADR kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 12,100 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu DR REDDYS LABORATORIES LTD ADR finden Sie auf Wallstreet Online.