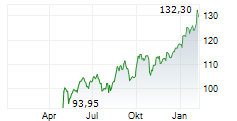
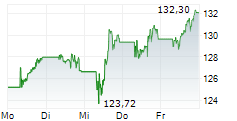
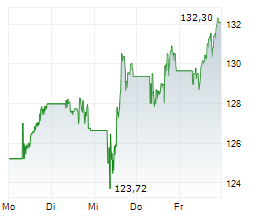
BASEL (dpa-AFX) - Novartis (NVS) announced that the U.S. Food and Drug Administration has approved Rhapsido (remibrutinib) as an oral treatment for adults with chronic spontaneous urticaria or CSU who continue to experience symptoms despite H1 antihistamine therapy. Taken twice daily, Rhapsido offers a convenient, injection-free option that does not require lab monitoring. It is the first Bruton's tyrosine kinase inhibitor (BTKi) to receive FDA approval for CSU, marking a significant advancement in treatment for patients with persistent symptoms.
Novartis noted that it has completed regulatory submissions for Rhapsido for the treatment of CSU across many countries, including in the European Union, Japan, and China, with priority review granted in China.
The company said that Remibrutinib is also in clinical development for chronic inducible urticaria, food allergy, and hidradenitis suppurativa, further expanding Novartis' immunology portfolio.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News