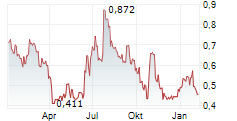
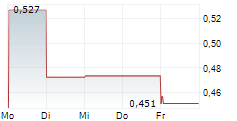
Mendus has announced a strategy update, unveiling plans to expand the application of vididencel beyond the current lead programme in acute myeloid leukaemia (AML), to chronic myeloid leukaemia (CML). Following encouraging preclinical results in CML, presented at ASH 2024, Mendus's clinical strategy will now pursue vididencel in this indication. A Phase Ia/Ib trial is being prepared to evaluate safety and feasibility, with first data expected in mid-2026. In parallel, in AML, Mendus has planned a Phase Ib trial to assess vididencel in combination with azacitidine and venetoclax in chemo-unfit patients, to run alongside the ongoing Phase II CADENCE trial (vididencel in combination with azacitidine only; now recruited 12 patients for part 1). Both trials are due to conclude in mid-2026. While a pivotal programme was previously planned from 2026 in AML, these new trials will now guide the go-to-market strategies in AML and CML. We note that this may lead to minor shifts in timelines/pathways and potentially incur additional costs, but this may be at least partially offset through a corporate reorganisation, and we believe the strategy has the potential to maximise the long-term value proposition of vididencel.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research