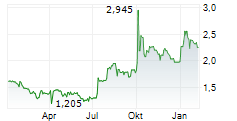
Herantis Pharma reported positive top-line results from the second part of its Phase Ib trial, evaluating lead candidate HER-096 in Parkinson's disease (PD) patients, meeting both the primary and secondary endpoints. Both 200mg and 300mg twice-weekly doses (administered for four weeks) were shown to be safe, well tolerated and achieved the predicted cerebrospinal fluid (CSF) exposure, confirming effective blood-brain barrier (BBB) penetration. We look forward to the full dataset and biomarker data to be released before end FY25 and believe that these top-line results provide pharmacological rationale for HER-096 to advance to Phase II efficacy studies. The 300mg twice-weekly dose has been deemed suitable for the Phase II trial in early-stage PD patients, expected to commence in 2026, potentially under a partnering agreement.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research