NEW YORK CITY (dpa-AFX) - Philip Morris International Inc. (PM), Wednesday announced that the company has urged U.S. Food and Drug Administration Advisory Committee to recommend continue marketing versions of its IQOS heated tobacco products in the U.S. as modified risk tobacco products.
The recommendation request was presented along with evidence to the FDA's Tobacco Products Scientific Advisory Committee.
The agency noted, 'Evidence continues to support the modified exposure claim that 'Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body's exposure to harmful or potentially harmful chemicals.'
Meanwhile, the FDA is in the process of completing its review of pending applications for IQOS ILUMA to reach and transition even more legal-age adults away from combustible cigarettes.
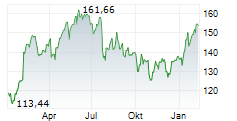
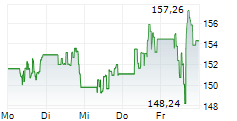
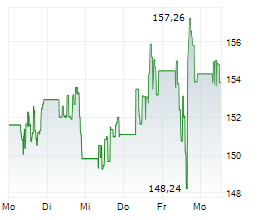
Currently, PM is trading at $154.30, down 0.16 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News