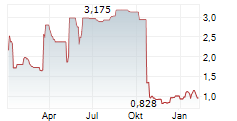
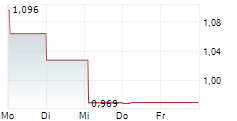
Xbrane Biopharma AB ("Xbrane"), a leading Swedish biosimilar developer, announce that the U.S. Food and Drug Administration (FDA) has issued a Complete Response Letter (CRL) to the Company's Biologics License Application (BLA) for its ranibizumab biosimilar candidate for treatment of retinal disorders. The CRL refers to observations following a re-inspection of one of Xbrane's contract manufacturers.
Xbrane re-submitted its BLA in December 2024 following a CRL received in April 2024 due to observations following inspections at its contract manufacturers production sites.
FDA conducted re-inspections during Q3 2025 of both production sites involved in production of drug substance and drug product respectively. Thorough evidence on corrective actions to the respective observations was submitted by both production sites in due time to the FDA. Xbrane has during the weekend received a CRL from FDA stating unresolved observations following the inspection at one of the production sites without further specification. No other issues related to the BLA were mentioned by the FDA in the CRL.
Xbrane and its contract manufacturer are now awaiting further communication from FDA, typically received by the production site within few days of issuing the CRL, to better understand the approvability hurdles. Xbrane will work together with the production site to resolve the issues mentioned, and to allow a re-submission of the BLA as soon as possible.
Xbrane's Lucentis biosimilar has been approved by EMA and MHRA since November 2022 and has been benefiting patients in related regions since March 2023. Close to 200,000 vials have been shipped to end-users without any issues.
"We are very disappointed about the FDA's decision to issue a CRL to our BLA. We believe US patients and payors would significantly benefit from a more cost-efficient alternative to existing approved treatments against retinal disorders and we are committed to work swiftly towards a re-submission of the BLA," commented Xbrane's CEO Martin Amark.
Xbrane currently does not have more information to be able to judge timing of re-submission but hope to gain further clarity during the coming week. Xbrane will therefore use the upcoming webcast related to the Q3 report on October 24th to discuss and answer related questions.
Contacts
Martin Åmark, CEO
E: martin.amark@xbrane.com
Jane Benyamin, CFO/IR
E: jane.benyamin@xbrane.com
About Us
Xbrane Biopharma AB develops biological drugs based on a patented platform technology that provides significantly lower production costs compared to competing systems. Xbrane has a portfolio of biosimilar candidates targeting EUR 23 billion in estimated annual peak sales of the respective reference product. The lead candidate Ximluci® is granted market authorization approval in Europe and was launched during 2023. Xbrane's head office is in Solna, just outside Stockholm. Xbrane is listed on Nasdaq Stockholm under the ticker XBRANE. For more information, visit www.xbrane.com
This information is information that Xbrane Biopharma is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact persons set out above, at 2025-10-19 20:00 CEST.