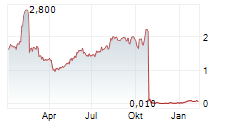
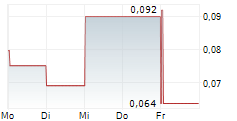
Guard Therapeutics (publ) today announced top-line results from the company's Phase 2b POINTER study evaluating the investigational drug candidate RMC-035 as a kidney-protective therapy in patients undergoing open-heart surgery. The study did not meet its primary and key secondary efficacy endpoints.
"The results from the POINTER study are disappointing, given previous findings of efficacy for RMC-035 and the significant unmet medical need in preventing irreversible kidney damage after open-heart surgery," said Tobias Agervald, CEO of Guard Therapeutics. "While the data do not support further development of RMC-035, we remain committed to fully understanding the study outcome and evaluating next steps."
The Phase 2b POINTER study did not demonstrate a statistically significant difference between RMC-035 and placebo in the primary endpoint, change in estimated glomerular filtration rate (eGFR) from baseline to Day 90. The placebo-adjusted change in eGFR for RMC-035 at Day 90 was -2.76 mL/min/1.73 m² (90% Confidence Interval [CI]: -6.10 to 0.58; p=0.17). Similarly, the key secondary endpoint, Major Adverse Kidney Events at Day 90 (MAKE90), was not met. The common relative risk for MAKE90 was 0.89 (90% CI: 0.48 to 1.66; p=0.76).
RMC-035 was generally well tolerated, and no safety concerns were identified.
The complete data set from POINTER is expected to be available within approximately two weeks. However, the company does not anticipate that additional analyses will change the overall outcome of the study.
Guard Therapeutics will host an open web call on October 27 at 2:00 p.m. CET to discuss the study results and respond to questions.
Link to the web conference: https://www.finwire.tv/webcast/guard-therapeutics/topline-results/
Upon completion of the full data review, the company's Board of Directors will initiate a strategic evaluation process to determine the optimal path forward for Guard Therapeutics. This review will include an assessment of the company's remaining resources, an integrated analysis of the full RMC-035 data set and the GTX peptide technology platform, as well as potential strategic alternatives. Further communication will be provided once the process has been completed.
For further information, please contact:
Tobias Agervald, CEO
Telephone: +46 8 670 65 51
E-mail: info@guardtherapeutics.com
About Guard Therapeutics
Guard Therapeutics is a Swedish clinical-stage biotechnology company that identifies and develops new therapies for diseases with a large unmet medical need, focusing on different forms of kidney disease. The company's candidate drugs are based on the endogenous protein alpha-1-microglobulin. Guard Therapeutics is listed on Nasdaq First North Growth Market Stockholm (ticker: GUARD).
Certified Adviser is Svensk Kapitalmarknadsgranskning AB, www.skmg.se.
About the POINTER study
The POINTER study is a randomized, double-blind, placebo-controlled Phase 2b trial of RMC-035 designed to evaluate its efficacy and safety as a kidney-protective treatment in open-heart surgery, and to determine the optimal dosing regimen and target patient population ahead of a registrational Phase 3 study.
The study includes a total of 170 patients randomized to two RMC-035 dose groups (60 mg and 30 mg) and a control group (placebo) in a 2:2:3 allocation. The primary efficacy endpoint is the change in renal function (eGFR) from baseline to Day 90 after surgery. Major Adverse Kidney Events (MAKE) at Day 90 after surgery is a secondary efficacy endpoint, defined as death, dialysis, or =25% loss of eGFR compared with baseline.
Data from the two RMC-035 dose groups will be pooled and compared with placebo in the primary efficacy analyses. Baseline kidney function was used as a stratification factor to ensure that patients with and without chronic kidney disease were evenly distributed across all treatment arms.
Patient recruitment for the study was completed during the second quarter of 2025.
About RMC-035
The company's lead candidate RMC-035 represents a completely new class of drugs (first-in-class) and consists of a recombinant and modified variant of the endogenous protein alpha-1-microglobulin. The investigational drug has the ability to protect cells and their mitochondria from damage caused by oxygen deprivation and elevated levels of the oxygen-binding and toxic protein heme. Favorable treatment effects of RMC-035 have been observed in several preclinical disease models. RMC-035 has a natural affinity for the kidneys and is primarily being developed as an intravenous kidney protective treatment for patients at high risk of developing acute kidney injury (AKI).
RMC-035 has obtained an Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration (FDA) for administration to patients in clinical studies. Additionally, RMC-035 has been granted Fast Track Designation by the FDA to reduce the risk of irreversible loss of kidney function, the need for dialysis treatment, or death after open-heart surgery in patients at elevated risk of AKI.
Results from the Phase 2 AKITA study, which enrolled 177 patients, demonstrated a statistically significant and clinically relevant beneficial effect of RMC-035 compared with placebo on long-term kidney outcomes in this patient population. Based on these results, a subsequent Phase 2b study, POINTER, was initiated.
In addition to its evaluation in open-heart surgery, RMC-035 has also been assessed in a Phase 1b clinical study in patients undergoing kidney transplantation.
This information is information that Guard Therapeutics is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact persons set out above, at 2025-10-26 11:45 CET.