- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 21.02. | Guard Therapeutics International AB Q4 Loss Decreases | 3 | RTTNews | ||
| 05.02. | Guard Therapeutics International AB: Guard Therapeutics Announces Changes to the Company's Management Team | 246 | GlobeNewswire (Europe) | Guard Therapeutics (publ) today announces changes to the company's management team, in light of the conclusion of the clinical program for the drug candidate RMC-035 and the ongoing strategic review... ► Artikel lesen | |
| 05.02. | Guard Therapeutics International AB: Guard Therapeutics Provides Status Update Regarding Ongoing Strategic Review | 127 | GlobeNewswire (Europe) | Guard Therapeutics International AB (publ) ("Guard Therapeutics" or the "Company") hereby provides a status update regarding the strategic review that the Company announced through a press release on... ► Artikel lesen | |
| 15.01. | Guard Therapeutics International AB: Guard Therapeutics Partners in EU Consortium For Alport Syndrome | 375 | GlobeNewswire (Europe) | Guard Therapeutics (publ) today announced its participation as a partner in a newly funded European research consortium under the 2025 Joint Transnational Call from the European Rare Diseases Research... ► Artikel lesen | |
| 11.12.25 | Guard Therapeutics International AB (publ) receives observation status | 188 | GlobeNewswire | On December 4, 2025, Guard Therapeutics International AB (publ) (the "Company") issued a press release with information that the Board of Directors of the Company had resolved to commence an exploratory... ► Artikel lesen | |
| 04.12.25 | Guard Therapeutics International AB: Guard Therapeutics Explores Strategic Alternatives | 162 | GlobeNewswire (Europe) | Guard Therapeutics (publ) ("Guard" or the "Company") today announces that the Board of Directors has resolved to commence an exploratory process regarding strategic alternatives, including identifying... ► Artikel lesen | |
| 04.12.25 | Guard Therapeutics International AB: Guard Therapeutics Provides Assessment of Full POINTER Study Results | 136 | GlobeNewswire (Europe) | Guard Therapeutics (public) ("Guard" or "the Company") today provides an assessment of the full results from the Phase 2b POINTER study evaluating RMC-035 in patients undergoing open-heart surgery.... ► Artikel lesen | |
| GUARD THERAPEUTICS INTERNATIONAL Aktie jetzt für 0€ handeln | |||||
| 13.11.25 | Guard Therapeutics International AB Q3 Loss Narrows | 1 | RTTNews | ||
| 26.10.25 | Guard Therapeutics International AB: Guard Therapeutics Announces Top-Line Results from Phase 2b POINTER Study | 461 | GlobeNewswire (Europe) | Guard Therapeutics (publ) today announced top-line results from the company's Phase 2b POINTER study evaluating the investigational drug candidate RMC-035 as a kidney-protective therapy in patients... ► Artikel lesen | |
| 05.10.25 | Guard Therapeutics International AB: Guard Therapeutics Announces New Positive Efficacy Results from Secondary Analyses of the Phase 2a AKITA Study | 189 | GlobeNewswire (Europe) | Guard Therapeutics (publ) today announced favorable efficacy results from additional analyses of the Phase 2a AKITA study with the investigational drug candidate RMC-035. The previously reported primary... ► Artikel lesen | |
| 11.09.25 | Guard Therapeutics International AB: Guard Therapeutics announces completion of data collection in Phase 2b POINTER study | 724 | GlobeNewswire (Europe) | Guard Therapeutics (publ) today announced that the last patient in its ongoing Phase 2b clinical trial, POINTER, has successfully completed the final scheduled follow-up visit 90 days after surgery... ► Artikel lesen | |
| 21.08.25 | Guard Therapeutics International AB: Guard Therapeutics publishes interim report January-June 2025 | 211 | GlobeNewswire (Europe) | The quarter marked a key milestone with the completion of patient recruitment for our Phase 2b POINTER study - ahead of schedule. The positive final safety review reinforces confidence in the trial... ► Artikel lesen | |
| 05.06.25 | Guard Therapeutics International AB: Guard Therapeutics announces completion of patient enrollment in Phase 2b POINTER study | 226 | GlobeNewswire (Europe) | Guard Therapeutics (publ) today announced that the last patient has been successfully enrolled in the company's ongoing Phase 2b clinical trial, POINTER, evaluating the drug candidate RMC-035 as a kidney-protective... ► Artikel lesen | |
| 27.05.25 | Guard Therapeutics International AB: Guard Therapeutics reports positive outcome from second planned safety review in the Phase 2b POINTER study | 266 | GlobeNewswire (Europe) | Guard Therapeutics today announced that an independent Data Safety Monitoring Committee (DSMC) has completed the second and final planned review of safety data from the ongoing Phase 2b clinical trial... ► Artikel lesen | |
| 25.03.25 | Guard Therapeutics International AB: Two-thirds of patients enrolled in Guard Therapeutics' Phase 2b POINTER study | 447 | GlobeNewswire (Europe) | Guard Therapeutics announced today that it has reached another key milestone in its ongoing clinical development program. Two-thirds of the approximately 160 planned patients have now been enrolled... ► Artikel lesen | |
| 14.03.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 14.03.2025 | 2.001 | Xetra Newsboard | Das Instrument OBD SE0000514705 OBDUCAT AB B SK 8 EQUITY wird cum Kapitalmassnahme gehandelt am 14.03.2025 und ex Kapitalmassnahme am 17.03.2025 The instrument OBD SE0000514705 OBDUCAT AB B SK 8 EQUITY... ► Artikel lesen | |
| 10.03.25 | Guard Therapeutics International AB: The Board of Directors of Guard Therapeutics has resolved on a previously announced rights issue of approximately SEK 150 million | 392 | GlobeNewswire (Europe) | THIS PRESS RELEASE MAY NOT BE MADE PUBLIC, PUBLISHED OR DISTRIBUTED, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, BELARUS, HONG KONG, JAPAN, CANADA, NEW ZEALAND, RUSSIA... ► Artikel lesen |
17 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| NOVONESIS | 48,980 | -1,96 % | Novozymes Reports Climb In Q4 Bottom Line | BRUSSELS (dpa-AFX) - Novozymes (NVZMF.PK) reported earnings for its fourth quarter that Increases, from the same period last yearThe company's earnings came in at EUR122.8 million, or EUR1.25... ► Artikel lesen | |
| GENMAB | 241,00 | -4,97 % | Genmab A/S - admittance to trading and official listing of new shares due to employee warrant exercise | The share capital of Genmab A/S has been increased. The admittance to trading and official listing will take effect as of 27 February 2026 in the ISIN below.
ISIN:
DK0010272202
Name:
Genmab
Volume... ► Artikel lesen | |
| ARBUTUS BIOPHARMA | 3,956 | -1,30 % | Arbutus Biopharma Corporation: Arbutus Reports Third Quarter 2025 Financial Results and Provides Corporate Update | Strong financial position with cash, cash equivalents and marketable securities of $93.7M Moderna litigation U.S. trial scheduled for March 2026;Favorable claim construction ruling in Pfizer-BioNTech... ► Artikel lesen | |
| VERICEL | 30,000 | -1,32 % | Vericel Corporation: Vericel Reports Fourth Quarter and Full-Year 2025 Financial Results | Total Revenue of $276.3 Million, with MACI Revenue Growth of 21% to $239.5 Million Net Income Growth of 59% to $16.5 Million Fourth Quarter Total Revenue and MACI Revenue Growth of 23% Record Fourth... ► Artikel lesen | |
| MOLECULIN BIOTECH | 2,350 | 0,00 % | Moleculin Biotech, Inc.: Moleculin MIRACLE Trial Delivers 40% Preliminary Blinded CRc Rate (n=30) | Preliminary blinded CR rate showed 67% improvement over historical cytarabine response Roughly 35% of the subjects treated to date represent ventoclax regimen failuresFirst 45 subjects treated on... ► Artikel lesen | |
| MUSTGROW BIOLOGICS | 0,310 | -1,59 % | MustGrow Biologics und die Partnerschaft mit Bayer: Darum steht die Aktie erst am Anfang | Die globale Landwirtschaft steht vor einem Paradox. Sie muss mehr Menschen ernähren, aber mit weniger chemischen Mitteln. Weltweit sind in 162 Ländern inzwischen 460 Pestizide verboten oder eingeschränkt.... ► Artikel lesen | |
| SENSEI BIOTHERAPEUTICS | 29,810 | 0,00 % | Sensei Biotherapeutics vergibt Aktienoptionen an 17 neue Mitarbeiter | ||
| BIOXCEL THERAPEUTICS | 1,378 | -0,86 % | BioXcel Therapeutics Planning to Submit sNDA This Month Seeking FDA Approval for At-Home Use of IGALMI | NEW HAVEN, Conn., Jan. 07, 2026 (GLOBE NEWSWIRE) -- BioXcel Therapeutics, Inc. (Nasdaq: BTAI), a biopharmaceutical company built on artificial intelligence ("AI") to develop transformative medicines... ► Artikel lesen | |
| REGENXBIO | 7,750 | -1,90 % | Regenerative Medizin im Aufbruch: NurExone Biologic, Regenxbio und Lineage Cell Therapeutics setzen auf unterschiedliche Zukunftstechnologien | ||
| AKEBIA | 1,070 | -1,11 % | Akebia outlines 2026 pipeline expansion and expects Vafseo data catalysts in $1B dialysis market | ||
| ZEVRA THERAPEUTICS | 7,400 | -5,13 % | Zevra Therapeutics Announces Details for Q4 and Full Year 2025 Financial Results Call | ||
| FENNEC PHARMACEUTICALS | 7,150 | 0,00 % | Fennec Pharmaceuticals Inc.: Fennec Pharmaceuticals Announces Investigator-Sponsored Trial to Be Conducted by City of Hope in Metastatic Testicular Germ Cell Tumors | - City of Hope to Evaluate PEDMARK- for Reducing Ototoxicity in Adult Men with Stage II-III Metastatic Testicular Germ Cell Tumors - - Initiation of Study Reflects Growing Clinical Interest in Addressing... ► Artikel lesen | |
| CORVUS PHARMACEUTICALS | 15,400 | +0,52 % | Corvus Pharmaceuticals, Inc.: Corvus Pharmaceuticals Announces Closing of Upsized Public Offering of Common Stock and Full Exercise of the Underwriters' Option to Purchase Additional Shares, Generating Gross Proceeds of Approximately $201M | SOUTH SAN FRANCISCO, Calif., Jan. 23, 2026 (GLOBE NEWSWIRE) -- Corvus Pharmaceuticals, Inc. (Nasdaq: CRVS), a clinical-stage biopharmaceutical company, today announced the closing of an upsized underwritten... ► Artikel lesen | |
| CARTESIAN THERAPEUTICS | 6,100 | +1,67 % | Cartesian Therapeutics, Inc.: Cartesian Therapeutics Highlights Recent Progress and Outlines 2026 Outlook | Enrollment on track in Phase 3 AURORA trial of Descartes-08 in myasthenia gravis IND application for Descartes-08 in myositis accepted by FDA; seamless adaptive clinical trial offering potential... ► Artikel lesen | |
| NANOBIOTIX | 26,800 | +1,13 % | Nanobiotix S.A.: NANOBIOTIX to Join CAC Mid 60 and SBF 120 Indices on Euronext Paris | PARIS and CAMBRIDGE, Mass., Dec. 15, 2025 (GLOBE NEWSWIRE) -- NANOBIOTIX (Euronext: NANO -- NASDAQ: NBTX - the "Company''), a late-stage clinical biotechnology company pioneering physics-based approaches... ► Artikel lesen |
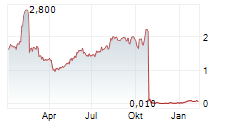
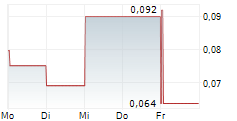
GUARD THERAPEUTICS INTERNATIONAL AB gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell -3,58 % bei einem Aktienkurs von 0,054 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GUARD THERAPEUTICS INTERNATIONAL AB finden Sie auf Wallstreet Online.