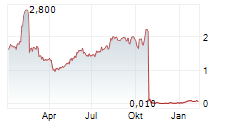
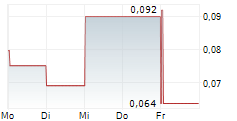
Guard Therapeutics (publ) ("Guard" or the "Company") today announces that the Board of Directors has resolved to commence an exploratory process regarding strategic alternatives, including identifying potential candidates for a merger or reverse takeover (RTO) of the Company. Guard believes that its listing on Nasdaq First North Growth Market in Stockholm, together with its cash position, may represent significant value for potential counterparties.
In parallel with this process, the Company continues its business development efforts and evaluation of alternative strategies for the preclinical GTX platform, which is considered to have substantial potential value that is not currently reflected in the Company's market valuation or has the right conditions to be developed independently in a public environment. At the same time, the Company has implemented cost-cutting activities to preserve resources and strengthen its ability to pursue these strategic alternatives
Comment from the Chairman of the Board
"As part of our strategic review, the Board sees it as a natural step to evaluate the possibilities of a merger or reverse takeover. Our listing and financial position can create value in such a process, while we continue to see strong potential in the GTX platform. It is important for us to ensure that this potential is realized in the best possible way for both patients and shareholders," says Johan Bygge, Chairman of Guard Therapeutics.
Current operations
Guard Therapeutics focuses on developing new drug candidates based on the endogenous protein alpha-1-microglobulin (A1M). The most advanced project, RMC-035 - a recombinant and modified variant of human A1M - has been evaluated in an extensive clinical program and has received Fast Track Designation from the FDA for reducing the risk of irreversible loss of kidney function, need for dialysis, or death following open-heart surgery in patients at increased risk of acute kidney injury.
Two large placebo-controlled Phase 2 studies, AKITA and POINTER, have been conducted. The AKITA study (Phase 2a, 177 patients) demonstrated a statistically significant and clinically relevant beneficial effect of RMC-035 compared with placebo on long-term kidney function outcomes. Based on these results, the POINTER study (Phase 2b, 170 patients) was carried out. The outcome was recently communicated and, despite encouraging signals in the lower dose group, the Company assesses that the results are not sufficiently strong to justify continued development of RMC-035. This assessment is primarily based on considerations related to the acute treatment regimen rather than a lack of relevance of the A1M mechanism in humans.
In parallel, the Company has developed GTX peptides, short and modified fragments of the A1M protein with retained pharmacological properties. Similar to RMC-035, these peptides have shown robust treatment effects in several preclinical disease models, but with the advantage that they can be administered as chronic therapy. This opens up broader potential applications. Clinical data with optimized dosing of RMC-035 demonstrate favorable effects on biomarkers and a reduced risk of acute kidney injury (AKI) during the active treatment phase. These results provide further support for the GTX peptides, which are considered capable of transferring similar effects to chronic disease settings with continuous treatment.
Strategic review
Guard Therapeutics is currently conducting a broad strategic review aimed at maximizing long-term value creation. With the discontinuation of the main program with RMC-035, in accordance with the separate press release issued on December 4, 2025, the GTX platform remains - an innovative peptide-based technology with potential in, among other areas, chronic kidney disease (CKD). However, the Company assesses that its current cash position is insufficient to advance the program to value-enhancing milestones without significant capital contributions. Further development of the platform is therefore considered optimal in collaboration with an external partner or in a private setting. The Company intends to continue discussions with pharmaceutical companies that have shown interest in RMC-035 and the GTX platform. If necessary, the GTX peptides may be spun out into a private company or excluded from the rights in connection with a potential acquisition or RTO.
In parallel, the Board of Directors has resolved to initiate an exploratory process to identify a growth company with an ambition for listing or a need for additional capital, where a merger or reverse acquisition could represent a possible path forward. Guard assesses that the Company's listing on Nasdaq First North Growth Market in Stockholm, together with its infrastructure, is attractive for the right type of target company.
The alternative solutions now under evaluation will be weighed against a potential liquidation of the Company. Should no strategic alternatives materialize within a reasonable timeframe, the Board intends to recommend delisting from Nasdaq First North Growth Market and voluntary liquidation, whereby available liquid funds would be distributed to shareholders. The Board intends to keep the market continuously informed of material events in the process, but emphasizes that at present there is no guarantee that any transaction will be completed. Decisions regarding a potential merger, reverse acquisition, delisting, or liquidation will be made by the shareholders at a future general meeting.
For further information, please contact:
Tobias Agervald, CEO
Telephone: +46 8 670 65 51
E-mail: info@guardtherapeutics.com
About Guard Therapeutics
Guard Therapeutics is a Swedish clinical-stage biotechnology company that identifies and develops new therapies for diseases with a large unmet medical need, focusing on different forms of kidney disease. The company's candidate drugs are based on the endogenous protein alpha-1-microglobulin. Guard Therapeutics is listed on Nasdaq First North Growth Market Stockholm (ticker: GUARD).
Certified Adviser is Svensk Kapitalmarknadsgranskning AB, www.skmg.se.
This information is information that Guard Therapeutics is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact persons set out above, at 2025-12-04 19:50 CET.