NEW BRUNSWICK (dpa-AFX) - Halozyme Therapeutics, Inc. (HALO), Friday announced that Johnson & Johnson has received U.S. Food and Drug Administration approval of a new indication for DARZALEX Faspro as a single agent treatment of adult patients with high-risk smoldering multiple myeloma.
The approval is based on the findings from the AQUILA study, which evaluated the efficacy and safety of DARZALEX Faspro.
Co-formulated with the company's drug delivery technology ENHANZE, Halozyme expects the approval to further strengthen DARZALEX Faspro's role as a cornerstone therapy across all stages of multiple myeloma.
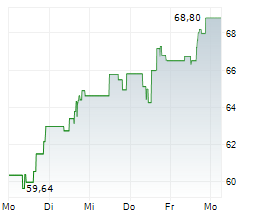
Currently, HALO is trading at $68.65, up 0.38 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News