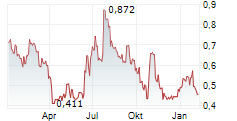
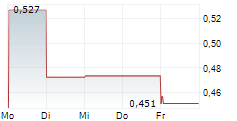
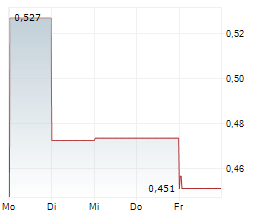
Mendus has reported its Q325 results, following its recently announced renewed strategy to broaden the potential for its lead cancer vaccine, vididencel, to include both acute myeloid leukaemia (AML) and chronic myeloid leukaemia (CML). Mendus has implemented organisational changes that should at least partially offset new expected clinical trial expenses. Key data readouts for its clinical trials in AML and CML are anticipated in mid-2026, and will represent a series of important inflection points for the company. In terms of its Q325 results, Mendus reported an operating loss of SEK20.4m for the quarter, 10% lower than Q324 (SEK22.7m), with the decline mainly attributed to reduced R&D costs for the technology transfer to NorthX Biologics in the year (SEK13.0m in Q325 versus SEK16.2m in Q324). We are reviewing our assumptions based on Mendus's new strategy and planned clinical programmes, and will follow up shortly with an updated discussion of Mendus's most recent financial performance and updated estimates.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research