BASEL (dpa-AFX) - Novartis Pharma AG (NVS) said Tuesday that the FDA has approved Itvisma for the treatment of children two years and older, as well as teens and adults living with spinal muscular atrophy with a confirmed mutation in the survival motor neuron 1 (SMN1) gene.
Spinal muscular atrophy (SMA) is a rare genetic neuromuscular disease caused by a mutation or deletion of the SMN1 gene. The SMN1 gene is responsible for producing most of the SMN protein the body needs for muscle function, including breathing, swallowing, and basic movement.
Itvisma is designed as a single, one-time intrathecal injection to address the genetic root cause of SMA by providing a functional copy of the human SMN1 gene to improve motor function through sustained SMN protein expression.
Also known as onasemnogene abeparvovec-brve, Itvisma is set to be launched in the U.S. this December. Its approval makes it the first and only gene-replacement therapy available for a broad population of SMA.
The company already markets a gene therapy for SMA called Zolgensma, which received U.S. approval in May 2019. Delivered as a one-time intravenous (IV) infusion, it is indicated for use in children under two years old who have spinal muscular atrophy (SMA) with bi-allelic mutations in the SMN1 gene.
For the first nine months of 2025, Zolgensma generated $925 million in global revenue, representing a 3% decline compared with the same period the previous year.
Approximately 9,000 people in the US live with SMA. According to Fortune Business Insights website report updated in November 2025, the global spinal muscular atrophy treatment market size stood at $1.72 billion in 2018 and is projected to reach $31.89 billion by 2032, exhibiting a CAGR of 21.8% during the forecast period.
'The FDA's approval of intrathecal onasemnogene abeparvovec is a game-changing advance, expanding the use of transformational gene replacement therapy for SMA across age groups,' said John W. Day, MD, PhD, Professor of Neurology and Paediatrics, Director, Division of Neuromuscular Medicine at Stanford University School of Medicine, and Co-Director of Stanford's Neuro IGNITE Centre.
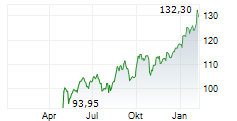
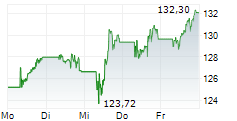
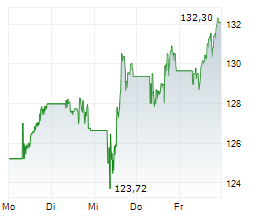
NVS has traded in a range of $96.06 to $134 in the last 1 year. The stock closed yesterday's trading at $126.54, down 0.60%. In premarket trading today, NVS is up 1.14% at $127.98.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News