NEW YORK CITY (dpa-AFX) - Valneva SE (VALN) announced positive final immunogenicity and safety data from its Phase 2 study, VLA15-221, evaluating the Lyme disease vaccine candidate VLA15. The study results demonstrated a strong anamnestic immune response and a favorable safety profile six months after administration of a third booster dose (month 48). These findings were consistent across all age groups, reinforcing the potential benefits of an annual vaccination ahead of each Lyme disease season.
Pfizer and Valneva entered into a collaboration agreement in April 2020 for the development and commercialization of VLA15. This partnership aims to advance the vaccine candidate toward broader availability, addressing the growing need for effective Lyme disease prevention.
There are currently no approved human vaccines for Lyme disease, and VLA15 has advanced the furthest in clinical development, with all vaccinations completed in the pivotal VALOR Phase 3 trial.
Valneva noted that its results further validate the use of the three-dose vaccination schedule and a yearly booster dose, already included in the Phase 3 protocols.
The safety and tolerability profile of VLA15 six months after the third booster dose was similar to the profile observed after previous booster doses. No safety concerns were observed by the independent DMC in any vaccination or age group.
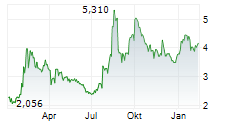
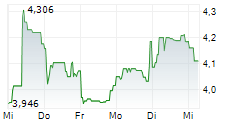
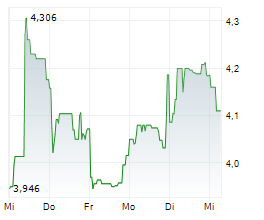
VALN closed Tuesday's regular trading at $8.74, down $0.08 or 0.91%. In overnight trading, the stock rose to $9.15, gaining $0.41 or 4.69%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News