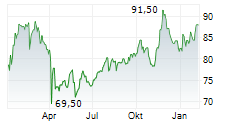
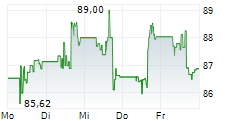
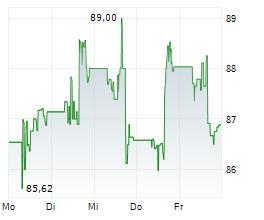
WASHINGTON (dpa-AFX) - Medtronic (MDT) announced that it has received FDA clearance for its Hugo robotic-assisted surgery (RAS) system in urologic procedures, marking a significant milestone in its surgical innovation portfolio. The Hugo system's modular design, integration with the Touch Surgery digital ecosystem, and Medtronic's unique ability to serve across open, laparoscopic, and robotic modalities position the company to expand its presence in the fast-growing robotic surgery market.
The clearance enhances Medtronic's competitive edge, supports adoption of minimally invasive care, and strengthens long-term growth opportunities. With differentiated technology and training capabilities, Medtronic is well-placed to drive value creation for healthcare providers and shareholders alike.
Outside the U.S., the Hugo RAS system has been used in tens of thousands of urologic, gynecologic, and general surgery procedures in more than 30 countries across 5 continents. Medtronic intends to expand the Hugo RAS system's use in the U.S. to additional surgical specialties over time, with indications for general and gynecologic surgical procedures expected to follow the initial urology clearance.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News