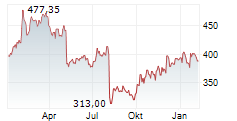
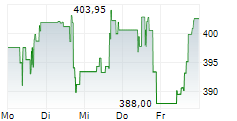
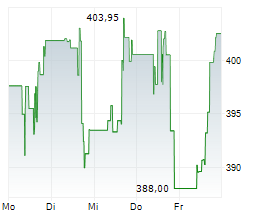
CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) announced new data from pivotal studies of CASGEVY (exagamglogene autotemcel), demonstrating the transformative potential of the therapy in children ages 5-11 years living with severe sickle cell disease (SCD) or transfusion-dependent beta thalassemia (TDT). These findings mark the first presentation of clinical data in this younger patient population.
The efficacy and safety outcomes observed in children ages 5-11 were consistent with the durable and positive benefit-risk profile already established in patients 12 years of age and older. This reinforces CASGEVY's potential as a groundbreaking therapy across age groups.
Vertex also announced its plans to initiate global regulatory submissions for CASGEVY in children ages 5-11 during the first half of 2026. This milestone underscores the company's commitment to expanding access to transformative therapies for younger patients.
CASGEVY is currently approved for eligible individuals ages 12 years and older with SCD or TDT in multiple regions, including the United States, Great Britain, the European Union, Saudi Arabia, Bahrain, Kuwait, Qatar, Canada, Switzerland, and the United Arab Emirates. The latest results, along with longer-term data in patients 12 years and older, will be presented at the upcoming American Society of Hematology (ASH) Annual Meeting.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News