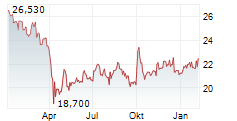
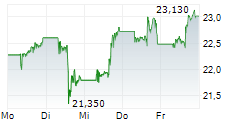
NEW YORK CITY (dpa-AFX) - Pfizer, Inc. (PFE) announced Tuesday it has inked an exclusive global collaboration and license agreement with YaoPharma, a subsidiary of global healthcare company Shanghai Fosun Pharmaceutical (Group) Co., Ltd., for the development, manufacturing and commercialization of YP05002, a small molecule glucagon-like peptide 1 (GLP-1) receptor agonist currently in Phase 1 development for chronic weight management.
Under the terms of the agreement, YaoPharma will complete an ongoing YP05002 Phase 1 clinical trial and grants Pfizer an exclusive license to further develop, manufacture and commercialize YP05002 worldwide.
YaoPharma will receive an upfront payment of $150 million and is eligible to receive milestone payments associated with certain development, regulatory and commercial milestones up to $1.935 billion, as well as tiered royalties on sales, if approved.
Pfizer plans to conduct combination studies of YP05002 with its glucose-dependent insulinotropic polypeptide receptor (GIPR) antagonist PF-07976016 currently in Phase 2 development and with other small molecules in its pipeline.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News