WASHINGTON (dpa-AFX) - Alnylam Pharmaceuticals (ALNY) on Wednesday said it plans to expand its manufacturing facility in Norton, Massachusetts, with an investment of about $250 million. The expansion is intended to support a fully dedicated, siRNA (small interfering RNA) enzymatic-ligation manufacturing facility.
As part of the project, Alnylam said its next-generation enzymatic ligation platform, siRELIS, has been accepted into the U.S. Food and Drug Administration's Emerging Technology Program, which facilitates early engagement with regulators on innovative manufacturing approaches. The platform has been demonstrated through pilot-scale production of zilebesiran, under study for reducing the risk of major adverse cardiovascular events in patients with hypertension, and nucresiran, in development for transthyretin-mediated amyloidosis.
The Norton facility, which opened in 2021, currently supports both clinical and early commercial manufacturing of siRNA drug substances. Construction on the expansion is underway, with the new capabilities expected to be fully operational by late 2027.
'At this pivotal time with our expanding pipeline of RNAi therapeutics, Alnylam is accelerating development of siRNA manufacturing and changing what's possible in a single facility,' said Yvonne Greenstreet, CEO of Alnylam. 'This advance will be a critical enabler in scaling our pipeline to include potential treatments for diseases such as hypertension, type 2 diabetes, and obesity.'
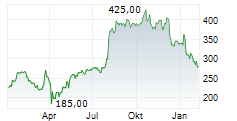
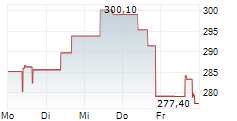
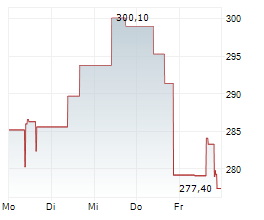
On Tuesday, Alnylam shares closed at $391.17, down 0.08%.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News