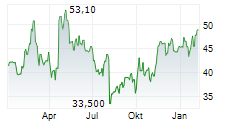
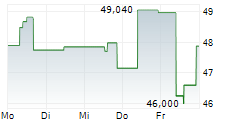
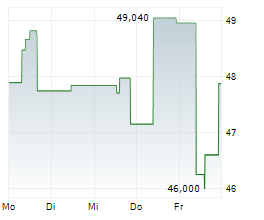
TOKYO (dpa-AFX) - Chugai Pharmaceutical Co., Ltd. (CHGCF.PK) on Monday announced that Japan's Ministry of Health, Labour and Welfare (MHLW) has approved Tecentriq Intravenous Infusion for an additional indication of unresectable thymic carcinoma.
The approval is based on results from the phase II MARBLE study conducted in Japan, which evaluated the efficacy and safety of Tecentriq in combination with carboplatin and paclitaxel as a first-line treatment for this patient group.
Since its launch in Japan in April 2018, Tecentriq has been approved for six other tumor types, including extensive-stage small cell lung cancer, non-small cell lung cancer, breast cancer, hepatocellular carcinoma, alveolar soft part sarcoma, and extranodal NK/T-cell lymphoma, nasal type.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News