WASHINGTON (dpa-AFX) - Edwards Lifesciences Corp. (EW) on Tuesday said its SAPIEN M3 mitral valve replacement system received U.S. Food and Drug Administration (FDA) approval for the treatment of mitral regurgitation (MR), a condition in which the heart's mitral valve does not close properly.
The SAPIEN M3 transcatheter mitral valve replacement (TMVR) system is indicated for the treatment of symptomatic moderate-to-severe or severe MR in patients. It is also approved for symptomatic mitral valve dysfunction associated with mitral annular calcification (MAC) in patients ineligible for surgery or TEER therapy.
The system received CE Mark in April 2025, making it the world's first approved transfemoral TMVR system.
Edwards Lifesciences' FDA-approved portfolio of transcatheter mitral and tricuspid therapies also includes the PASCAL Precision mitral valve repair syste
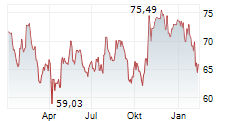
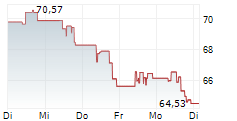
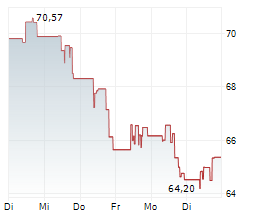
On Monday, Edwards Lifesciences ' shares closed at $86.53, up 1.02%.m and the EVOQUE tricuspid valve replacement system.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News