LONDON (dpa-AFX) - HUTCHMED (China) Limited (HCM, HCM.L, 0013.HK) announced that the Phase III registration portion of the ESLIM-02 clinical trial evaluating sovleplenib, a novel spleen tyrosine kinase (Syk) inhibitor, in adult patients with warm antibody autoimmune hemolytic anemia (wAIHA) in China has successfully met its primary endpoint. The study demonstrated a durable hemoglobin (Hb) response rate between weeks 5 and 24 of treatment, marking a significant milestone in the development of sovleplenib for this rare autoimmune blood disorder.
Autoimmune hemolytic anemia ('AIHA') is an autoimmune disorder characterized by the destruction of red blood cells ('RBCs') due to the production of antibodies against RBC.
Sovleplenib is a novel, investigational, selective small molecule inhibitor for oral administration targeting the spleen tyrosine kinase, also known as Syk. Syk is a major component in B-cell receptor and Fc receptor signaling and is an established target for the treatment of multiple subtypes of B-cell lymphomas and autoimmune disorders.
HUTCHMED plans to submit the New Drug Application for sovleplenib for wAIHA to the China National Medical Products Administration (NMPA) in the first half of 2026.
In addition to wAIHA, sovleplenib is also being studied in immune thrombocytopenia or 'ITP'. Positive results from ESLIM-01, a Phase III trial in China of sovleplenib in patients with primary ITP, have been published in The Lancet Haematology. An NDA resubmission for sovleplenib for second-line ITP is planned in the first half of 2026.
HUTCHMED currently retains all rights to sovleplenib worldwide.
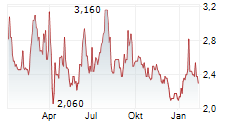
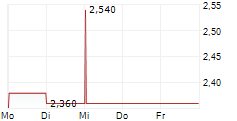
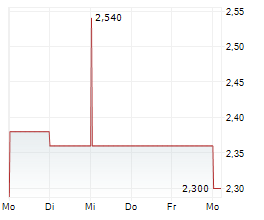
HCM closed Tuesday's regular trading session at $13.75, up $0.38 or 2.84% as of 4:00 PM EST. In after-hours trading, the stock continued to rise, reaching $14.02, an increase of $0.27 or 1.96% as of 7:47 PM EST.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News