STOCKHOLM (dpa-AFX) - Swedish Orphan Biovitrum AB (publ) (SOBI.ST) or Sobi announced topline results from the Phase 2a EMBRACE study evaluating Gamifant (emapalumab) for interferon-gamma (IFN?)-driven sepsis (IDS). The study demonstrated proof-of-concept, with observed improvements in both organ dysfunction and survival.
The research was conducted in collaboration with the Hellenic Institute for the Study of Sepsis (HISS). Based on the findings, Sobi and HISS plan to advance the development of emapalumab in IDS and will engage with regulatory authorities to determine the next clinical steps. An update will be provided in due course, and detailed data from the EMBRACE study will be presented at an upcoming medical conference.
Sepsis is a severe condition triggered by infection that can lead to organ failure and remains a leading global cause of mortality. A large study published in eBioMedicine in 2024 described different sepsis endotypes, highlighting the need for differentiated treatment strategies. Approximately 20% of patients were identified with the newly described IFN?-driven sepsis (IDS) endotype. IDS is characterized by elevated CXCL9 levels, detection of IFN?, and poor clinical outcomes, with a 28-day mortality rate ranging from 40% to 43%.
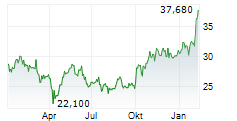
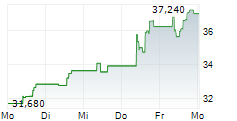
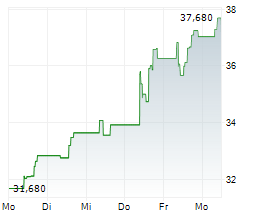
Swedish Orphan Biovitrum closed trading in Stockholm at SEK 337.60 , up SEK 3.80 or 1.14%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News