NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) announced positive results from Cohort 3 of the pivotal BREAKWATER trial, evaluating BRAFTOVI (encorafenib) in combination with cetuximab (ERBITUX) and FOLFIRI (fluorouracil, leucovorin, and irinotecan) in patients with previously untreated metastatic colorectal cancer (mCRC) harboring the BRAF V600E mutation.
The analysis revealed that the BRAFTOVI regimen with cetuximab and FOLFIRI achieved a confirmed objective response rate (ORR) of 64.4%, compared to 39.2% with standard-of-care treatment (FOLFIRI with or without bevacizumab). This improvement, assessed by blinded independent central review (BICR), was both clinically meaningful and statistically significant.
The estimated median duration of response was not yet determinable for patients treated with the BRAFTOVI regimen, while patients receiving FOLFIRI with or without bevacizumab showed a median duration of 7.0 months. Importantly, 57.4% of patients in the BRAFTOVI arm experienced responses lasting six months or longer, compared to 34.5% in the standard-of-care group.
Overall survival (OS) data were analyzed descriptively, showing a hazard ratio of 0.49 with a median follow-up of approximately 10 months for both arms. The BREAKWATER trial remains ongoing, with completion expected in 2027.
The safety profile of BRAFTOVI in combination with cetuximab and FOLFIRI was consistent with the known profiles of each agent, and no new safety signals were identified. The most common side effects (?15%) included nausea, diarrhea, vomiting, alopecia, anemia, decreased neutrophil count, decreased appetite, fatigue, neutropenia, skin hyperpigmentation, dry skin, asthenia, weight loss, arthralgia, palmar-plantar erythrodysaesthesia syndrome, rash, decreased white blood cell count, and constipation. Among patients receiving the combination regimen, 8.5% experienced adverse reactions that led to permanent discontinuation of BRAFTOVI.
BRAFTOVI in combination with cetuximab and FOLFIRI is an investigational regimen and is not currently approved for use. BRAFTOVI in combination with cetuximab and mFOLFOX6 received accelerated approval by the U.S. Food and Drug Administration (FDA) in December 2024 for patients with BRAF V600E -mutant mCRC based on a clinically meaningful and statistically significant improvement in confirmed ORR in treatment-naïve patients, one of the study's primary endpoints. Continued approval for this indication is contingent upon verification of clinical benefit.
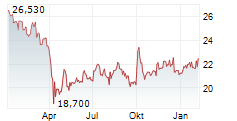
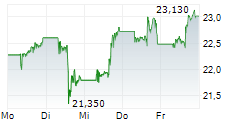
PFE closed trading on January 9 at $25.48, reflecting a gain of $0.19 or 0.75% during regular market hours. In after-hours trading, the stock slightly dipped to $25.47, down $0.01 or 0.04%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News