LONDON (dpa-AFX) - HUTCHMED (China) Limited (HCM) on Wednesday said results from the Phase III SACHI trial evaluating savolitinib in combination with osimertinib were published in The Lancet.
The SACHI study, conducted in China, assessed the combination compared to platinum-based doublet chemotherapy, in patients with epidermal growth factor receptor (EGFR) mutation-positive, MET-amplified non-small cell lung cancer (NSCLC) whose disease had progressed following first-line EGFR tyrosine kinase inhibitor therapy.
The trial met its primary endpoint, with an objective response rate (ORR) of 58% in the savolitinib plus osimertinib arm, compared with 34% in the chemotherapy arm. The disease control rate (DCR) was 89% versus 67%, while median duration of response (DoR) was 8.4 months versus 3.2 months, respectively.
The savolitinib and osimertinib combination was approved in China in June 2025.
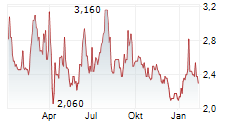
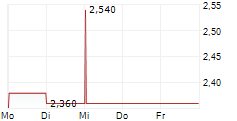
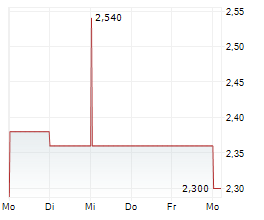
HUTCHMED shares rose more than 4% in after-market trading after closing up 0.13% at $14.95.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News