TOKYO (dpa-AFX) - Ionis Pharmaceuticals, Inc. (IONS) and Otsuka Pharmaceutical Co., Ltd. on Wednesday said the European Commission has approved Dawnzera to prevent repeated attacks of hereditary angioedema (HAE) in people aged 12 and older.
HAE is a rare genetic condition that can be life-threatening and causes sudden swelling in different parts of the body.
Otsuka has the exclusive rights to Dawnzera in Europe and the Asia-Pacific region. With the approval, Ionis will receive a $15 million milestone payment and tiered royalties of up to 30% on sales.
The approval is based on Phase 3 OASIS-HAE and OASISplus studies, which showed that Dawnzera effectively reduced HAE attacks.
Dawnzera was already approved in the U.S. in August 2025 for preventing HAE attacks in patients 12 and older.
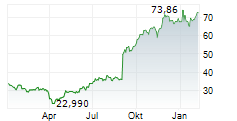
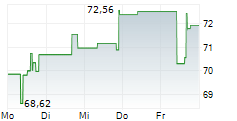
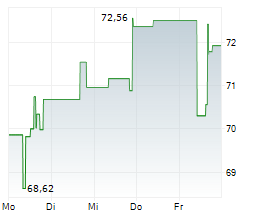
On Tuesday, Ionis Pharma shares closed at $77.53, up 1.57%.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News