TOKYO (dpa-AFX) - Takeda Pharmaceutical Company Limited (TAK), Thursday announced the U.S. availability of GAMMAGARD LIQUID ERC, which is approved as a replacement therapy for people two years of age and older with primary immunodeficiency.
GAMMAGARD LIQUID ERC is a ready-to-use liquid immunoglobulin therapy with an IgA content of less than or equal to 2 µg/mL in a 10 percent solution to be administered intravenously or subcutaneously.
'As the latest addition to our broad IG portfolio, GAMMAGARD LIQUID ERC underscores our ongoing dedication to providing differentiated therapeutic options to meet the varied needs of people with primary immunodeficiency, including those who require an option with low IgA content,' said Uthra Sundaram, senior vice president and head of Takeda's U.S. Plasma-Derived Therapies Business Unit.
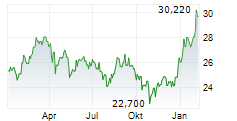
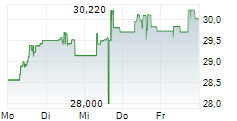
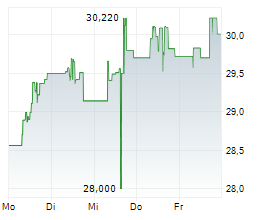
In the pre-market hours, TAK is trading at $15.97, down 0.73 percent on the New York Stock Exchange.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News