DJ Carvolix is born and funded
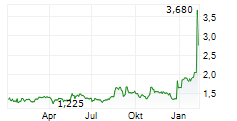
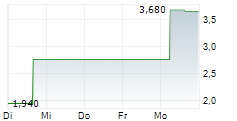
Affluent Medical Carvolix is born and funded 02-Feb-2026 / 07:30 CET/CEST Dissemination of a French Regulatory News, transmitted by EQS Group. The issuer is solely responsible for the content of this announcement. =---------------------------------------------------------------------------------------------------------------------- Affluent Medical officially becomes CARVOLIX Completion of the acquisitions of CARANX MEDICAL and ARTEDRONE 10mEUR first tranche of financing completed Aix-en-Provence, February 2, 2026 - 7:30 a.m. CET - Carvolix (formerly Affluent Medical) (ISIN: FR0013333077 - Ticker: AFME - "Carvolix" or the "Company"), a French commercial and clinical-stage medical technology company specializing in the international development and industrialization of breakthrough AI-driven mini-robots and biomimetic implants, to revolutionize interventional cardiology and the treatment of brain stroke is today announcing the completion of the acquisitions of Caranx Medical and Artedrone (the "Acquisitions"), for a closing price paid in Carvolix shares, to form a new, integrated MedTech company named Carvolix, as well as the definitive terms of the capital increases and the issuance of new shares (the "Financing" and, together with the Acquisitions, the "Transaction") pursuant to the use of the delegations granted by the general meeting held on January 30, 2026 (the "General Meeting"). A 10MEUR first tranche of Financing has been made available by funds managed by Truffle Capital and by Edwards Lifesciences at a subscription price of EUR2.34 per share (which represents a 18.8% premium versus the last closing share price). This strategic consolidation is designed to create a company for the 21st century interventional cardiologist - leveraging world leading technology in AI driven autonomous mini-robots with a mission to democratize complex, life-saving procedures. The combined platforms position Carvolix to accelerate radical innovation, expand its addressable market, and drive long term value creation. "We're applying our proven business builder model - uniting the capabilities of Truffle-founded companies to de-risk development, accelerate innovation, generate synergies and unlock value for shareholders" said Philippe Pouletty, M.D., CEO of Truffle Capital, founder of several successful biotech and medtech companies (including Abivax, Vexim, Symetis and Affluent Medical). He added: "We expect to make the cardiology catheterization lab as autonomous and efficient as an aircraft cockpit so that our radical innovations could potentially benefit to millions of patients worldwide." Carvolix will focus on revolutionizing cardiac valve replacement and brain stroke treatment, addressing major unmet medical needs in large markets with a total addressable value of EUR23 billion. Currently, only 17% of the 1.7 million patients annually eligible for Transcatheter Aortic Valve Implantation (TAVI) undergo the procedure, and only 5% of ischemic stroke patients (second cause of death, third cause of disability) receive mechanical thrombectomy. Similarly, just 4% of the four million patients with severe mitral valve regurgitation undergo surgery. "We are bringing together three extremely innovative and synergistic MedTech companies into one - with the goal of augmenting the cardiac catheterization lab to treat far more patients suffering from valve dysfunction and brain stroke" said Sebastien Ladet, CEO of Carvolix. "In addition, we will boost synergies in R&D and commercialization between the three companies to enable the development and delivery of additional products, such as a robotically delivered mitral valve." The combination of these companies unites deep expertise and R&D synergies across micro-robotics, AI, image guidance and biomimetic valve technologies - accelerating innovation and establishing a robust, sustained product development cadence. The first product launch is occuring in early 2026, with the TAVIPILOT software 'already cleared by the FDA, being introduced in the US. The Company plans to keep direct commercialization rights in Europe and seek partners in the US, Middle East, and Asia. "We are building a fantastic management team and board of directors to carry out our mission: a commercial stage MedTech leader dedicated to helping interventional cardiologists treat more patients around the world" said Liane Teplitsky, Executive Chair of the Board of Directors of Carvolix. Completion of the Acquisitions On December 19, 2025, the Company announced the upcoming acquisition of Caranx Medical and Artedrone, becoming Carvolix, pioneering cardiovascular therapies with AI driven autonomous mini-robots and innovative implants. On January 30, 2026, the Acquisitions have been formally completed and the consolidation of Caranx Medical and Artedrone with the pre-existing Affluent Medical entities into Carvolix is now effective. The terms and conditions of the Acquisitions are further detailed in the Company press release dated December 19, 2025. In particular, Carvolix made closing payments of respectively EUR16.6M and EUR11.4M, entirely in Carvolix shares, for the acquisition of Caranx Medical and Artedrone. Carvolix also acquired from Truffle BioMedTech CrossOver FPCI a current account against Artedrone for an amount of EUR1M plus accrued interests (at a rate of 8% per annum) (the "Current Account "). Truffle funds, acting as sellers in the context of the Acquisitions, entirely rolled-over the closing purchase price and the purchase price of the Current Account at a subscription price of EUR2.34 per ordinary share (i.e., the same price as for the Financing), resulting in the issuance of a total of 12,384,470 new ordinary shares. These 12,384,470 new ordinary shares were issued by the Board of Directors on January 30, 2026 pursuant to the delegations granted by the general meeting of the Company held on January 30, 2026, for a total subscription price of EUR28,979,659.80 offset with the closing purchase price and the purchase price of the Current Account. Initial Tranches of Financing On December 19, 2025, the Company further announced the launch of a concomitant Financing of up to EUR30M led by Truffle Capital and Edwards Lifesciences who undertook to make available to the Company a first tranche of EUR10M (the "First Tranche"). The Company will secure up to EUR300,000 from several business angels (the "Additional Tranche", and together with the First Tranche, the "Initial Tranches"). Following the adoption by shareholders of the appropriate resolutions at the General Meeting, the Board of Directors decided on January 30, 2026 to use the delegations granted by the General Meeting to proceed with the implementation of the Initial Tranches, consisting of 4,401,708 new ordinary shares at a subscription price of EUR2.34 per Financing Share (which represents a 18.8% premium versus the last closing share price). The purpose of the Initial Tranches of the Financing is to extend the horizon of the Company's cash position from December 2025 to the end of May 2026. The Initial Tranches of the Financing will allow the Company to pursue clinical and regulatory development for all its devices, and to position itself favorably as it embarks on the next steps of value creation. These steps include, but are not limited to, launching the commercialization in the US of TAVIPILOT Software, negotiating a strategic agreement with an industrial player to speed up the clinical trials and marketing of Artus, progress towards first in human for the robotic platform for stroke treatment and continue the development and clinical activities of Epygon. The allocation of the proceeds of the Initial Tranches between the different programs should be approximately as follows: 28% to TAVIpilot, 27% to Artus, 23% to structural heart devices (Kalios and Epygon), 22% to ARTE-DRONE. The financing required to pursue the combined Carvolix activities over the next 12 months, according to current development plans, is estimated at around EUR26M, of which EUR10.3M is secured through the Initial Tranches of the Financing. The Company expects to secure the remainder (i.e., an additional amount of EUR15.7M) from several international investors, with whom discussions are currently ongoing. The Company also expects to be able to further extend its cash runway through, among other things, the proceeds that would be generated from a potential partnership deal with regards to Artus, an artificial urinary sphincter currently in Carvolix's product portfolio. Conversion of the June 2025 convertible bonds On June 20, 2025, the Company issued EUR5.4M in convertible bonds to some of its main historical shareholders (the " Convertible Bonds"). In accordance with their terms, the Convertible Bonds are immediately and automatically payable in connection with the implementation of the Financing. Each bondholder has irrevocably undertaken to subscribe, by way of debt offset, to a capital increase concomitant to the First Tranche of the Financing, such that no cash reimbursement will be made in connection with the redemption of the Convertible Bonds. The ordinary shares issued in connection with the redemption of the Convertible Bonds are subscribed by the bondholders at a price of EUR1.872 per ordinary share (corresponding to a 20% discount). Following the adoption by shareholders of the appropriate resolutions at the General Meeting, the Board of Directors decided on January 30, 2026 to use the delegations granted by the General Meeting to proceed with the redemption of the Convertible Bonds and the issuance of 3,107,305 ordinary shares (including 2,605,384 ordinary shares to be issued to Truffle Medeor FPCI) at a subscription price of EUR1.872 per ordinary share (which represents a 5% discount versus the last closing share price). Impact of the Transaction on the share capital Taking into account the share issues resulting from (i) the Roll-Over, (ii) the Initial Tranches of the Financing, and (iii) the redemption of the Convertible Bonds, the shareholding structure of the Company will be as follows: The 19,893,483 shares issued will result in a dilution of 33.57% (on a non-diluted basis). Following such issuance, the Company's share capital will amount to EUR5,924,367.50 divided into 59,243,675 shares with a nominal value of EUR0.10. A shareholder holding 1.00% of the Company's share capital before the Transaction would therefore hold 0.66% of the capital after the completion of the Transaction. As part of the Transaction, it is reminded that up to an additional 14,283,985 shares could be issued in connection with the payment of earn-outs for the acquisition of Caranx Medical and Artedrone as well as with the subsequent tranches of the Financing (assuming up to 20MEUR in additional financing subscribed at a price of EUR2.34 per share), representing an additional dilution of 14% (on a non-diluted basis). In accordance with applicable provisions of the French Commercial Code, each subscriber of ordinary shares has abstained from voting on the resolution related to the removal of shareholders' preferential rights for its benefit at the General Meeting and, as the case may be, the representatives of the subscribers at the Board of Directors have abstained from participating to the relevant decisions of the Board of Directors. Impact of the Transaction on the shareholders' equity The share of equity, based on the Company's consolidated financial statements at June 30, 2025, would be as follows: Total equity (consolidated equity at June 30, 2025 in thousands of euros) 21.110EUR Share of equity before issuance of the 19,893,483 shares 0,54EUR Share of equity after issuance of the 19,893,483 shares 1,08EUR
Governance
As previously announced, the Board has decided to appoint Mrs. Liane Teplitsky as Executive Chair of the Board of Directors in replacement of Mr. Michel Therin, who will continue to contribute to the Board as director, effective as from the date of closing of the Transaction.
Advisors
Dechert LLP acted as legal advisor to the Company in connection with the Transaction.
Settlement and Delivery - Documentation
The ordinary shares issued in connection with (i) the Roll-Over, (ii) the Initial Tranches of the Financing, and (iii) the redemption of the Convertible Bonds, are expected to be admitted to trading on Euronext on February 4, 2026.
Such ordinary shares will be subject to an application for admission to trading on Euronext on the same trading line as the existing ordinary shares of the Company currently listed on Euronext, under the same ISIN code FR0013333077.
The Transaction is not subject to a prospectus requiring approval by the Financial Markets Authority (the "AMF"). However, in accordance with Article 1.5.b bis of Regulation (EU) 2017/1129 of the European Parliament and of the Council of June 14, 2017, as amended (the "Prospectus Regulation"), the Company will file with the AMF a document containing the information required in Annex IX of the Prospectus Regulation (the "Information Document"), with a view to the admission to trading on the regulated market of Euronext in Paris ("Euronext Paris") of the Shares to be issued in connection with the Transaction. The Information Document is not subject to a review by the AMF.
Risk factors
Members of the public should take note of the risk factors relating to Carvolix and its business, as presented in Chapter 3 of the 2024 Universal Registration Document filed with the AMF on April 30, 2025 under number D.25-0356, which is available free of charge on Carvolix's website (www.affluentmedical.com). The occurrence of all or some of these risks would be likely to have an adverse effect on the business activity, financial position, results, development, or outlook of Carvolix. Such events could have a material adverse effect on Carvolix's share price. Members of the public should particularly take note of the following risks:
-- Raising additional capital, including as a result of this Transaction or of further offerings to finance the
development or the commercialization of Carvolix's products, may cause dilution to the Company's shareholders,
restrict its operations or require it to relinquish rights to its products; -- Future sales of ordinary shares by existing shareholders or investors participating in the Transaction could
depress the market price of the Company's shares; -- The market price of the Company's shares can be subject to significant fluctuations and may decrease below the
issuance price retained in the context of the Transaction; -- Volatility and liquidity of the shares of the Company can be subject to significant fluctuations; -- The Company's management will have broad discretion over the use of the proceeds from the Financing and may apply
these proceeds in ways that may not result in an increase of the share price.
In particular, Carvolix has updated risk factor 3.4.1 "Liquidity risk" of the 2024 Universal Registration Document in order to take into account the Financing, as well as risk factor 3.4.3 "Dilution risk" of the 2024 Universal Registration Document (for more information about the dilution, please see the above capitalization table) to reflect that the exercise of all the dilutive instruments held by officers, directors and employees, the warrants issued to Kreos and the earn-outs granted in the context of the Acquisitions, would result in a dilution of 31% to existing shareholders on the basis of the Company's current share capital.
This press release does not constitute a prospectus as referred to in Regulation (EU) 2017/1129 of the European Parliament and of the Council of June 14, 2017, as amended, or an offer to the public.
About Carvolix
Carvolix is a French medical technologies company, commercial and clinical stage, founded by Truffle Capital, that aims to become a global leader in the treatment of structural heart diseases and brain strokes, world's leading causes of mortality and disability. Carvolix develops novel AI and imaging driven mini-robots that make complex procedures doble by interventional cardiologists, as well as biomimetics heart valves.
About Truffle Capital
Founded in 2001, Truffle Capital is an independent European Venture Capital firm specializing in disruptive technologies in the Life Sciences (Medtech and Biotech) and IT sectors (Fintech and Insurtech). Truffle Capital's mission is to support the creation and development of innovative companies capable of becoming the leaders of tomorrow, and it has notably founded Abivax.
Managed by Philippe Pouletty, M.D. and Bernard-Louis Roques, Co-founders and co-CEOs, Truffle Capital manages EUR500 million in assets. It has raised more than EUR1.2 billion since its creation and has supported more than 124 companies in the digital technology and life sciences sectors. Discover more at www.truffle.com and follow us on LinkedIn.
Contacts:
CARVOLIX SEITOSEI.ACTIFIN
Communication financière / relations presse financière
Sébastien Ladet Ghislaine GASPARETTO / Enora BUDET
CEO +33 (0)6 85 36 76 81 / +33 (0)6 72 17 84 60
investor@carvolix.eu ghislaine.gasparetto@seitosei-actifin.com / enora.budet@seitosei-actifin.com
MC SERVICES AG
PRIMATICE
Relations médias Europe
Relations médias France
Thomas Roborel de Climens Maximilian SCHUR / Julia BITTNER
+33 (0)6 78 12 97 95
+49 (0)211 529252 20 / +49 (0)211 529252 28
thomasdeclimens@primatice.com
affluent@mc-services.eu
Disclaimer
This press release contains forward-looking statements about Carvolix and its business. All statements other than statements of historical fact included in this press release, including, but not limited to, statements regarding Carvolix's financial condition, business, strategies, plans and objectives for future operations are forward-looking statements. Carvolix believes that these forward-looking statements are based on reasonable assumptions. However, no assurance can be given that the expectations expressed in these forward-looking statements will be achieved. These forward-looking statements are subject to numerous risks and uncertainties, including those described in Chapter 3 of the 2024 Universal Registration Document filed with the AMF on April 30, 2025 under number D.25-0356, which is available on the Company's website (www.affluentmedical.com), as well as the risks associated with changes in economic conditions, financial markets and the markets in which Carvolix operates. The forward-looking statements contained in this press release are also subject to risks that are unknown to Carvolix or that Carvolix does not currently consider material. The occurrence of some or all of these risks could cause the actual results, financial condition, performance or achievements of Carvolix to differ materially from those expressed in the forward-looking statements. This press release and the information contained herein do not constitute an offer to sell or subscribe for, or the solicitation of an order to buy or subscribe for, shares of Carvolix in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. The distribution of this press release may be restricted in certain jurisdictions by local law. Persons into whose possession this document comes are required to comply with all local regulations applicable
to this document.
This press release does not constitute a prospectus within the meaning of Regulation (EU) 2017/1129 of the European Parliament and of the Council of 14 June 2017 (the "Prospectus Regulation"). With respect to the member states of the European Economic Area (each, a "Relevant Member State"), no offer of the securities mentioned herein is made or will be made to the public in that Relevant Member State, except (i) to any legal person who is a qualified investor as defined in the Prospectus Regulation, (ii) to fewer than 150 natural or legal persons per Relevant Member State, or (iii) in other circumstances falling within Article 1(4) of the Prospectus Regulation; provided that none of these offers shall require the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Regulation. For the purposes of the foregoing, the expression "offer to the public" in any Relevant Member State has the meaning given to it in Article 2(d) of the Prospectus Regulation.
Solely for the purposes of each manufacturer's product approval process, the target market assessment in respect of the securities offered in the Financing has led to the conclusion that, in relation to the type of clients criteria, (i) the target market for the securities is eligible counterparties and professional clients, each as defined in Directive 2014/65/EU, as amended ("MiFID II"); and (ii) all channels for distribution of the securities offered in the Financing to eligible counterparties and professional clients are appropriate. Any person subsequently offering, selling or recommending the shares (a "distributor") should take into consideration the manufacturers' client type assessment; however, a distributor subject to MiFID II is responsible for undertaking its own target market assessment in respect of the shares offered in the Financing (by either adopting or refining the manufacturers' target market assessment) and determining appropriate distribution channels.
This press release has been prepared in French, German, Arabic and English. In the event of any discrepancy between the four versions of the press release, the French version shall prevail.
----------------------------------------------------------------------------------------------------------------------- Regulatory filing PDF file
File: Carvolix is born and funded
=------------------------------------------------------------------
Language: English
Company: Affluent Medical
320 avenue Archimède, Les pléiades III Bâtiment B
13100 Aix en Provence France
France
Phone: +33 4 42 95 12 20
E-mail: jerome.geoffroy@affluentmedical.com
Internet: https://www.affluentmedical.com/
ISIN: FR0013333077
Euronext Ticker: AFME
AMF Category: Inside information / Other releases
EQS News ID: 2269244
End of Announcement EQS News Service
=------------------------------------------------------------------------------------
2269244 02-Feb-2026 CET/CEST
Image link: https://eqs-cockpit.com/cgi-bin/fncls.ssp?fn=show_t_gif&application_id=2269244&application_name=news&site_id=dow_jones%7e%7e%7ebed8b539-0373-42bd-8d0e-f3efeec9bbed
(END) Dow Jones Newswires
February 02, 2026 01:30 ET (06:30 GMT)