NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) on Friday said the U.S. Food and Drug Administration (FDA) has granted Priority Review to the company's supplemental Biologics License Application (sBLA) for HYMPAVZI.
The application seeks approval to expand the use of HYMPAVZI to include treatment of hemophilia A or B patients aged 6 years and older with inhibitors, as well as pediatric patients aged 6 to 11 years with hemophilia A or B without inhibitors.
The FDA has set a Prescription Drug User Fee Act (PDUFA) action date in the second quarter of 2026.
The submission is supported by results from Phase 3 BASIS and Phase 3 BASIS KIDS trials.
If approved, HYMPAVZI would be the first preventive therapy for children aged 6 to 11 with hemophilia B that does not require clotting factors replacement, Pfizer said.
In the U.S., HYMPAVZI is currently approved for patients 12 years and older with hemophilia A without factor VIII inhibitors, or hemophilia B without factor IX inhibitors.
Additionally, the use of HYMPAVZI for patients aged 12 years and older with hemophilia A or B with inhibitors is under review by the European Medicines Agency.
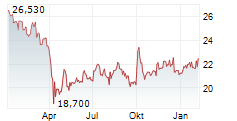
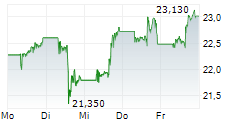
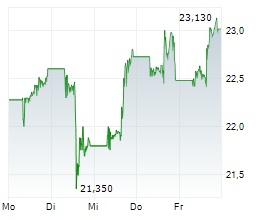
Pfizer shares closed 1.08% lower at $26.49 on Thursday.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News