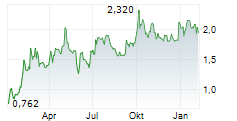
SynAct Pharma has completed patient recruitment for its Phase IIb ADVANCE trial (n=240) in rheumatoid arthritis (RA), while initiating the Phase II RESPIRE study in hospitalised with respiratory insufficiency due to viral infections (n=96). Together, these developments reinforce SynAct's dual clinical strategy for resomelagon in both chronic inflammatory disease and the acute hospital setting. While recruitment progress in the ADVANCE trial de-risks trial execution, database closure and analysis timelines imply top-line ADVANCE data will likely move from our previously anticipated Q126 window into Q226. We expect top-line readouts from the ADVANCE trial to remain the key catalyst for partnering discussions and valuation inflection. We will present our adjusted estimates following SynAct's FY25 results on 18 February 2026.Den vollständigen Artikel lesen ...
© 2026 Edison Investment Research