Herantis Pharma Plc
Company release, inside information, 11 February 2026 at 23:45 (EET)
Inside information: Herantis Pharma Plc successfully completes a directed share issue raising EUR 4.2 million
THE INFORMATION CONTAINED IN THIS RELEASE IS NOT FOR PUBLICATION OR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN OR INTO AUSTRALIA, CANADA, HONG KONG, JAPAN, SINGAPORE, SOUTH AFRICA OR THE UNITED STATES OR IN ANY OTHER JURISDICTION IN WHICH PUBLICATION OR DISTRIBUTION WOULD BE UNLAWFUL.
Herantis Pharma Plc ("Herantis" or the "Company"), a clinical-stage company developing disease-modifying therapies to stop the progression of Parkinson's disease, has carried out an offering of new shares (the "New Shares") in a directed share issue to institutional and other qualified investors (the "Directed Issue"). Herantis raises gross proceeds of EUR 4.2 million in the Directed Issue.
The Company issues a total of 2,409,000 New Shares in the Directed Issue, representing approximately 10.00 per cent of all issued shares prior to the Directed Issue and approximately 9.09 per cent of all issued shares following the Directed Issue. The total number of issued shares in the Company after the Directed Issue will be 26,503,817.
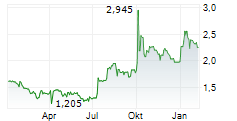
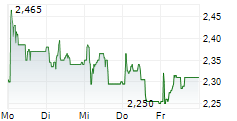
The subscription price is EUR 1.75 per New Share, corresponding to a discount of approximately 15.7 per cent compared to the volume-weighted average trading price on 11 February 2026 on Nasdaq First North Growth Market Finland. The subscription price shall be recorded in the invested unrestricted equity reserve.
"This EUR 4.2 million financing strengthens our position and provides flexibility as we continue preparations for our Phase 2 proof-of-concept study of HER-096", said Antti Vuolanto, CEO of Herantis Pharma. "These funds will also support our ongoing discussions with prospective pharma partners as we identify the optimal route to execute the Phase 2 trial and generate shareholder value. We are pleased to have completed the financing with certainty despite the recent volatility in the Finnish life sciences and biotechnology capital markets. In this environment, the support from existing and new investors underlines confidence in our program's potential as a disease-modifying therapy for Parkinson's disease and our progress to date. We look forward to updating shareholders further in due course."
The Directed Issue was based on the authorization given to the board of directors by the Company's annual general meeting of 24 April 2025.
Most of the New Shares (ISIN code FI4000087861) will be registered with the trade register on or about 13 February 2026 and they are expected to be ready for delivery to the investors through Euroclear Finland Ltd on or about 16 February 2026. The remainder of the New Shares are expected to be registered later in February 2026.
Herantis intends to make an application for the admission to trading of the New Shares on Nasdaq First North Growth Market Finland. Trading in most the New Shares is expected to commence on Nasdaq First North Growth Market Finland on or about 16 February 2026 with the remaining New Shares to be listed shortly thereafter, subject to the respective listing applications being approved.
Herantis intends to use the net proceeds from the Directed Issue for preparation of the Phase 2 clinical trial of HER-096, advancing partnering discussions, and general corporate purposes. In preparing the Directed Issue, the Board of Directors has assessed several different financing options in addition to which their feasibility has been assessed on several occasions by the management of the Company. After careful consideration, the Board of Directors has determined that raising the funds by way of Directed Issue in derogation of the shareholders' pre-emptive rights is a better alternative for the Company's shareholders than a rights issue.
The Company needs additional funding within a timeframe in which it is not possible to execute a rights issue. Compared to the Directed issue, rights issue would entail a significantly longer execution time and thereby increased market exposure and a higher potential risk of affecting the share price negatively particularly in a recently volatile market environment. The cost of carrying out the Directed Issue is deemed to be lower than in a rights issue where, among other things, there would be a risk that a rights issue would not be fully subscribed and significant underwriting commitments from an underwriting syndicate would possibly have to be procured.
Further, the Board of Directors has a positive view on an increased shareholding in the Company among institutional investors and other qualified investors because of their nature as long-term investors, who are able to offer substantial capital investments cost-efficiently. Consequently, and taking into account the Company's plan to use the funds raised for development of HER-096, when comparing a potential rights issue and the Directed Issue and their respective implications on execution cost and timeline, the Directed Issue allows the Company to obtain financing on terms and in a timetable that will be more beneficial than terms that would otherwise be available. Taking into account the above-mentioned matters, weighty financial reasons exist for derogating from the shareholders' pre-emptive rights, considered as a whole.
The subscription price of the New Shares has been determined based on the investor meetings arranged by the company's management together with the lead managers, Swedbank AB (publ) and UB Asset Management Ltd, and on negotiations regarding the subscription commitments. The determination of subscription price on market terms has been verified by the lead managers having sought to gather indications of interest to subscribe from a number of institutional investors in addition to which negotiations on subscription commitments have been concluded by independent parties. The subscription price reflects a discount that takes into account the recent developments in the Finnish life sciences and biotechnology capital markets.
The silent period preceding the Company's financial statements release commenced in early February 2026, meaning that the Directed Issue has been resolved upon and is executed during the silent period. The board of directors has assessed that executing the Directed Issue during the silent period is more beneficial for the Company than waiting until the silent period ends on 5 March 2026, as delaying the Directed Issue would have exposed the fundraising to changes in market conditions and therefore it is reasoned to deviate from the Company's disclosure policy and its requirement on silent period in order to facilitate investor discussions and the Directed Issue. No persons discharging managerial responsibilities within the Company or their closely associated persons participated in the Directed Issue and no previously unpublished information was disclosed to investors during the investor discussions held in the context of the Directed Issue.
Swedbank AB (publ) and UB Asset Management Ltd act as the lead managers in the Directed Issue. Krogerus Attorneys Ltd is acting as the legal counsel to the Company.
Herantis Pharma Plc
The Board of Directors
For more information, please contact:
Tone Kvåle, CFO
Tel: +47 915 19576
Email: ir@herantis.com
Certified Advisor:
UB Corporate Finance Ltd
Tel.: +358 9 25 380 225
E-mail: ubcf@unitedbankers.fi
About Herantis Pharma Plc
Herantis Pharma Plc is a clinical-stage biotechnology company developing disease-modifying therapies for Parkinson's disease. The Company's lead product, HER-096, is a first-in-class small peptide that combines the neuroprotective mechanism of cerebral dopamine neurotrophic factor (CDNF), with the convenience of subcutaneous administration. In a Phase 1b clinical trial, HER 096 was shown to be generally safe and well tolerated in Parkinson's disease patients. Herantis plans to advance HER-096 into a Phase 2 clinical trial in 2026 to evaluate efficacy, safety and tolerability in early-stage Parkinson's patients.
Herantis is listed on the Nasdaq First North Growth Market Finland.
Company website: www.herantis.com