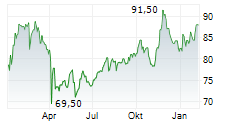
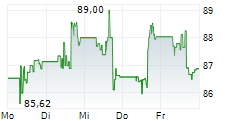
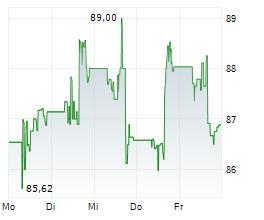
DUBLIN (dpa-AFX) - Medtronic plc (MDT), a healthcare technology firm, announced Friday that the U.S. Food and Drug Administration (FDA) cleared the Stealth AXiS surgical system, a next-generation first integrated robotics platform that brings planning, navigation, and robotics together into a single, intelligent system for spine surgery.
The Stealth AXiS system is cleared for spine procedures in the U.S., with an underlying architecture designed to support future cranial and ENT applications, pending 510(k) clearance.
The Stealth AXiS system creates a clear pathway for adoption by combining familiar navigation workflows with a modular robotic design, allowing institutions to deploy what they need today and enabling future expansion across procedures, specialties, and care settings.
A key innovation of the Stealth AXiS system is LiveAlign segmental tracking, an industry-first capability that allows surgeons to visualize anatomic motion, surgical adjustments, and patient alignment in real time during spine surgery, without the need for repeated imaging.
This capability helps reduce reliance on manual steps and workflow disruption, supporting more consistent execution of patient-specific surgical plans.
As a cornerstone of Medtronic's AiBLE smart ecosystem, the Stealth AXiS system enables a more intuitive and seamless flow of information across the surgical continuum.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News