| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
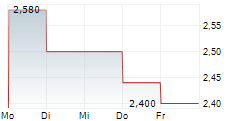
| 2,800 | 2,820 | 02.03. | |
| 2,720 | 2,900 | 02.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 06.02. | Imunon stock price target raised to $25 from $14 at H.C. Wainwright | 10 | Investing.com | ||
| IMUNON Aktie jetzt für 0€ handeln | |||||
| 05.02. | Imunon-Aktie legt zu: Restrukturierung schärft Fokus auf entscheidende Phase-3-Studie | 9 | Investing.com Deutsch | ||
| 05.02. | Imunon, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 07.01. | Imunon, Inc. - 8-K, Current Report | 7 | SEC Filings | ||
| 07.01. | Brookline Capital senkt Kursziel für Imunon wegen Verwässerung auf 16 Dollar | 10 | Investing.com Deutsch | ||
| 07.01. | Brookline Capital lowers Imunon stock price target to $16 on offering dilution | 3 | Investing.com | ||
| 31.12.25 | Imunon, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 30.12.25 | IMUNON raises $7 million in registered direct offering | 3 | Investing.com | ||
| 30.12.25 | Imunon, Inc.: IMUNON Announces Pricing of $7.0 Million Registered Direct Offering Priced At-The-Market Under NASDAQ Rules | 4 | GlobeNewswire (USA) | ||
| 13.11.25 | Imunon, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 10 | SEC Filings | ||
| 13.11.25 | Imunon GAAP EPS of -$1.16 beats by $0.65 | 7 | Seeking Alpha | ||
| 13.11.25 | Imunon, Inc.: IMUNON Reports Third Quarter 2025 Financial Results and Provides Business Update | 808 | GlobeNewswire (Europe) | R&D Day showcased remarkable Investigator optimism, continued strengthening supportive data, and significant progress with Phase 3 OVATION 3 Study in pursuit of first frontline immunotherapy for advanced... ► Artikel lesen | |
| 13.11.25 | Imunon, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 13.11.25 | Imunon, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 12.11.25 | Exploring Imunon's Earnings Expectations | 4 | Benzinga.com | ||
| 12.11.25 | Imunon Q3 2025 Earnings Preview | 3 | Seeking Alpha | ||
| 10.11.25 | Imunon, Inc.: IMUNON Public Webcast Highlights Resilience and Innovation in Pursuit of the First Approved Immunotherapy for Ovarian Cancer | 3 | GlobeNewswire (USA) | ||
| 10.11.25 | Imunon, Inc.: IMUNON R&D Day Showcases Clinical Progress of Its Novel Immunotherapy, Phase 3 Trial and Significant Potential for Women with Ovarian Cancer | 4 | GlobeNewswire (USA) | ||
| 04.11.25 | Imunon, Inc.: IMUNON Announces Webcast of In-Person R&D Day Highlighting Progress on OVATION 3 Study in Pursuit of First Frontline Immunotherapy for Advanced Ovarian Cancer | 5 | GlobeNewswire (USA) | ||
| 23.10.25 | IMUNON präsentiert auf SITC-Tagung neue Daten zur Eierstockkrebs-Therapie | 16 | Investing.com Deutsch |
1 2 3 Weiter >>
45 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 50,78 | -1,05 % | Fresenius: Weiteres Wachstum in Sicht - Aktie bärenstark | Am kommenden Mittwoch (25. Februar) wird der Gesundheitskonzern Fresenius die Zahlen für das vergangene vierte Quartal respektive Gesamtjahr 2025 vorlegen. Der Markt rechnet sowohl umsatz- als auch... ► Artikel lesen | |
| FRESENIUS MEDICAL CARE | 39,840 | +1,58 % | EQS-CMS: Fresenius Medical Care AG: Veröffentlichung einer Kapitalmarktinformation | EQS Zulassungsfolgepflichtmitteilung: Fresenius Medical Care AG
/ Bekanntmachung nach Art. 5 Abs. 1 lit. b) der Verordnung (EU) Nr. 596/2014
Fresenius Medical Care AG: Veröffentlichung... ► Artikel lesen | |
| CARL ZEISS MEDITEC | 25,800 | -5,43 % | Carl Zeiss Meditec AG: ZEISS launches Collaborative Care application to strengthen continuity of care; new solution offers on-premises and cloud-based options | Built on the ZEISS Health Data Platform, ZEISS Collaborative Care offers eye care professionals a flexible way to collaborate - whether as a standalone cloud application or as an integrated... ► Artikel lesen | |
| UNITEDHEALTH | 252,50 | +1,71 % | Former Optum CEO Heather Cianfrocco to depart UnitedHealth Group | ||
| GERRESHEIMER | 16,120 | +2,68 % | DAX fester, Bitcoin tiefer: FMC, MTU, IBM, Telekom, Infineon, Airbus, SAP, Gerresheimer im ... | Der DAX hat am Montag Verluste einstecken müssen. Aus dem Handel ging er mit einem Minus von 1,1 Prozent bei 24.991,97 Punkten. Der Start in den Dienstag dürfte hingegen etwas freundlicher sein. Der... ► Artikel lesen | |
| DRAEGERWERK | 89,50 | 0,00 % | Draegerwerk AG & Co. KGaA: Zollentlastung bereits eingepreist, Aufwärtspotenzial wird kleiner, Kursziel erhöht; auf HALTEN gestuft. | Die Zollerlassungen in den USA bieten einen inkrementellen Unterstützungseffekt für die Draegerwerk, jedoch wird der Nutzen voraussichtlich schrittweise erfolgen. Während bestimmte Zölle aufgehoben... ► Artikel lesen | |
| MEDTRONIC | 83,94 | +1,56 % | Medtronic Launches MiniMed Go Smart MDI System In Europe | WASHINGTON (dpa-AFX) - Medtronic (MDT) announced the commercial launch in Europe of the MiniMed Go Smart MDI system with the Simplera sensor. The launch will be rolled out gradually across Europe... ► Artikel lesen | |
| INTUITIVE SURGICAL | 426,60 | +0,15 % | Im Krankenhaus auf Aktiensuche: Paul Hartmann, Technogym und Intuitive Surgical | Ein paar Dinge habe ich nach meinen Krankenhaus-Aufenthalten im November, Dezember und Januar gelernt...
...zum Beispiel: Es ist egal, wie schlecht es dir geht, es geht immer noch jemandem schlechter... ► Artikel lesen | |
| THERMO FISHER | 434,95 | -1,37 % | THERMO FISHER SCIENTIFIC INC. - 10-K, Annual Report | ||
| TELADOC HEALTH | 4,400 | -0,89 % | Teladoc Health Q4 Loss Narrows | WASHINGTON (dpa-AFX) - Teladoc Health, Inc. (TDOC) on Wednesday reported a fourth-quarter net loss of $25.1 million, or $0.14 per share, compared to $48.4 million or $0.28 per share last year.Fourth-quarter... ► Artikel lesen | |
| STRATEC | 20,650 | -1,90 % | EQS-PVR: STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: STRATEC SE
STRATEC SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide... ► Artikel lesen | |
| RHOEN-KLINIKUM | 12,800 | -0,78 % | EQS-PVR: RHÖN-KLINIKUM AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung | EQS Stimmrechtsmitteilung: RHÖN-KLINIKUM Aktiengesellschaft
RHÖN-KLINIKUM AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung
20.02.2026... ► Artikel lesen | |
| HIMS & HERS HEALTH | 14,040 | +14,38 % | Marge kollabiert: Hims stürzt nach Prognose ab - Novo Nordisk bleibt der Brandbeschleuniger | © Foto: Thomas Fuller - SOPA Images via ZUMA Press WireTrotz starker Q4-Zahlen reißt der neue Ausblick ein Loch in die Story. Hohe Ausgaben, ein schwaches erstes Quartal und der Novo-Konflikt setzen... ► Artikel lesen | |
| SIEMENS HEALTHINEERS | 41,370 | -2,41 % | RBC stuft Siemens Healthineers auf 'Outperform' | NEW YORK (dpa-AFX Analyser) - Die kanadische Bank RBC hat Siemens Healthineers mit einem Kursziel von 55 Euro auf "Outperform" belassen. Die Nervosität der Anleger vor einem möglichen Verkauf des Diagnostikgeschäfts... ► Artikel lesen | |
| GENEDX | 74,20 | -6,98 % | These Analysts Slash Their Forecasts On GeneDx After Q4 Results |
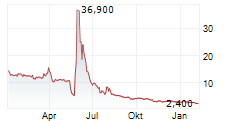
Das Unternehmen IMUNON INC kann der Branche Gesundheitswesen zugeordnet werden. Die relative Kursveränderung beträgt aktuell +3,25 % bei einem Aktienkurs von 2,540 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu IMUNON INC finden Sie auf Wallstreet Online.