- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 30.01. | OKYO Pharma präsentiert Studienergebnisse zu Urcosimod auf jährlicher ASCRS-Tagung | 1 | Investing.com Deutsch | ||
| 30.01. | OKYO Pharma to present urcosimod NCP study results at ASCRS meeting | 1 | Investing.com | ||
| 30.01. | OKYO Pharma Announces Acceptance of Urcosimod Phase 2 Study Results for Presentation at Prestigious ASCRS Annual Meeting | 295 | GlobeNewswire (Europe) | LONDON and NEW YORK, Jan. 30, 2026 (GLOBE NEWSWIRE) -- OKYO Pharma Limited (Nasdaq: OKYO), a clinical-stage biopharmaceutical company developing investigational therapies for the treatment of neuropathic... ► Artikel lesen | |
| 30.01. | OKYO Pharma Ltd - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| OKYO PHARMA Aktie jetzt für 0€ handeln | |||||
| 29.01. | H.C. Wainwright reiterates Buy rating on OKYO Pharma stock with $7 price target | 1 | Investing.com | ||
| 28.01. | OKYO Pharma meldet erfolgreiches FDA-Treffen zur klinischen Studie mit Urcosimod | - | Investing.com Deutsch | ||
| 28.01. | OKYO Pharma erhält grünes Licht der FDA für entscheidende NCP-Studie | - | Investing.com Deutsch | ||
| 28.01. | OKYO Pharma receives FDA alignment on Phase 2b/3 trial for NCP treatment | 2 | Investing.com | ||
| 28.01. | OKYO Pharma Announces Successful Type C Meeting with the FDA | 219 | GlobeNewswire (Europe) | FDA confirms Phase 2b/3 clinical design, including primary endpoint, sample size and development approachLONDON and NEW YORK, Jan. 28, 2026 (GLOBE NEWSWIRE) -- OKYO Pharma Limited (Nasdaq: OKYO), a... ► Artikel lesen | |
| 26.01. | OKYO Pharma erhält FDA-Sondergenehmigung für Urcosimod | 1 | Investing.com Deutsch | ||
| 26.01. | OKYO Pharma stock gets FDA compassionate use approval for urcosimod | 2 | Investing.com | ||
| 23.01. | OKYO Pharma LTD: FDA Approves Compassionate Use of Urcosimod (0.05%) for the Treatment of Neuropathic Corneal Pain | 313 | GlobeNewswire (Europe) | LONDON and NEW YORK, Jan. 23, 2026), a clinical-stage biopharmaceutical company developing investigational therapies for the treatment of neuropathic corneal pain (NCP) and for inflammatory eye diseases... ► Artikel lesen | |
| 23.01. | OKYO Pharma Ltd - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| 06.01. | H.C. Wainwright reiterates Buy rating on OKYO Pharma stock at $7 target | 1 | Investing.com | ||
| 05.01. | OKYO Pharma appoints Robert Dempsey as new CEO | 1 | Investing.com | ||
| 05.01. | OKYO Pharma CEO Gary Jacob Transitions To CDO; Appoints Robert Dempsey As CEO | 2 | RTTNews | ||
| 05.01. | OKYO Pharma ernennt Robert J. Dempsey zum neuen Chief Executive Officer | 1 | Investing.com Deutsch | ||
| 05.01. | OKYO Pharma Appoints Industry Veteran Robert J. Dempsey as Chief Executive Officer | 207 | GlobeNewswire (Europe) | Accomplished industry leader Robert J. Dempsey brings extensive commercialization and ophthalmic expertise Gary S. Jacob, Ph.D., will transition to Chief Development Officer and continue to serve on... ► Artikel lesen | |
| 05.01. | OKYO Pharma Ltd - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 19.12.25 | OKYO Pharma: Management läutet Eröffnungsglocke der NASDAQ in New York | 2 | Investing.com Deutsch |
1 2 3 Weiter >>
59 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| IDEXX LABORATORIES | 548,40 | +0,55 % | ROUNDUP: IDEXX Laboratories Guides FY26 In Line With Estimates | WASHINGTON (dpa-AFX) - While reporting financial results for the fourth quarter on Monday, pet healthcare provider IDEXX Laboratories, Inc. (IDXX) initiated its earnings and revenue guidance... ► Artikel lesen | |
| BIOXCEL THERAPEUTICS | 1,298 | +1,25 % | BioXcel Therapeutics kündigt Meilenstein- und Halteprämien für Führungskräfte an | ||
| ADAPTIMMUNE THERAPEUTICS | 0,016 | 0,00 % | Adaptimmune Therapeutics PLC: Adaptimmune Announces Changes to Board and Executive Leadership Team | Philadelphia, Pennsylvania and Oxford, United Kingdom--(Newsfile Corp. - November 17, 2025) - Adaptimmune Therapeutics plc (OTC Pink: ADAPY) ("Adaptimmune" or the "Company") today announced changes... ► Artikel lesen | |
| VOYAGER THERAPEUTICS | 3,126 | +0,51 % | Voyager Therapeutics, Inc.: Voyager Reports Third Quarter 2025 Financial and Operating Results | - Momentum building around tau: expect VY7523 clinical data and VY1706 clinical entry in 2026 - - Sharpened focus on multi-modality pipeline with introduction of Voyager NeuroShuttle discovery... ► Artikel lesen | |
| ANAVEX LIFE SCIENCES | 3,617 | +4,15 % | ANAVEX LIFE SCIENCES CORP kurz vor dem entscheidenden Ausbruch | ||
| CELLAVISION | 14,480 | +2,40 % | CellaVision: Solid Quarter driven by Strong Performance in Americas | Organic sales growth:Q4, 2025: 12.2% (-7.4)EBITDA margin:Q4, 2025: 33% (33)Oktober 1st - December 31st, 2025Net sales increased by 5.6% (-7.0) to SEK 197 m (187).Sales increased organically by 12.2%... ► Artikel lesen | |
| PRECISION BIOSCIENCES | 3,000 | -6,83 % | Precision BioSciences: Sind das jetzt Super-Schnäppchen? | Die Aktie von Precision BioSciences ist eines der wenigen Biotech-Papiere, die in diesem Jahr keinen Höhenflug erlebten. Im Gegenteil: Die Aktie hat ohne schlechte Unternehmensnews in den letzten Monaten... ► Artikel lesen | |
| ZYMEWORKS | 19,700 | +2,07 % | Zymeworks Inc.: Zymeworks Announces Participation in Upcoming Investor Conferences | VANCOUVER, British Columbia, Jan. 27, 2026 (GLOBE NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME), a biotechnology company managing a portfolio of licensed healthcare assets, while developing a diverse pipeline... ► Artikel lesen | |
| VTV THERAPEUTICS | 26,200 | +5,65 % | vTv Therapeutics Inc. - 8-K, Current Report | ||
| QUOIN PHARMACEUTICALS | 6,120 | 0,00 % | Quoin Pharmaceuticals, Inc.: Quoin Pharmaceuticals Announces Submission to Japanese MHLW for Orphan Drug Designation for QRX003 in Netherton Syndrome | Confirmation Received from MHLW That QRX003 Qualifies for Both Orphan Drug Designation and Fast Track Regulatory Review Status Quoin Initiating the Establishment of a Japanese Subsidiary to Facilitate... ► Artikel lesen | |
| NEUPHORIA THERAPEUTICS | 4,070 | +1,75 % | Neuphoria Therapeutics, Inc.: Neuphoria Provides Facts, Exposes False Narratives in Response to Lynx1 Fictions | BURLINGTON, Mass., Dec. 01, 2025 (GLOBE NEWSWIRE) -- Neuphoria Therapeutics Inc. ("Neuphoria" or the "Company") (NASDAQ: NEUP), a clinical-stage biotechnology company dedicated to developing therapies... ► Artikel lesen | |
| PLIANT THERAPEUTICS | 0,940 | -3,09 % | Pliant Therapeutics, Inc.: Pliant Therapeutics Announces Interim Data from PLN-101095 in Patients with Immune Checkpoint Inhibitor-Refractory Advanced Solid Tumors | One complete response and three partial responses observed in heavily pretreated ICI-secondary refractory patients in high dose cohorts Deep and durable ongoing responses with median time on treatment... ► Artikel lesen | |
| MIRA PHARMACEUTICALS | 0,950 | -5,00 % | MIRA Pharmaceuticals Initiates Final Cohort of Ketamir-2 Phase 1 Clinical Study as Company Prepares for Phase 2a Study | Company to Participate in BIO Partnering Summit and Present Phase 1 Ketamir-2 Data at American Association for Cancer Research (AACR)Annual Meeting MIAMI, FLORIDA / ACCESS Newswire / February 3, 2026... ► Artikel lesen | |
| NUVATION BIO | 5,705 | +1,42 % | Nuvation Bio stock maintains Buy rating at B.Riley on Ibtrozi sales momentum | ||
| ONCONETIX | 1,170 | -4,10 % | Onconetix, Inc.: Joint Press Release: Onconetix and Ocuvex Announce Mutual Termination of Merger Agreement | CINCINNATI, OH & FORT LEE, NJ, Sept. 26, 2025 (GLOBE NEWSWIRE) -- Onconetix, Inc. (Nasdaq: ONCO), a commercial-stage biotechnology company focused on innovative solutions for men's health and oncology... ► Artikel lesen |
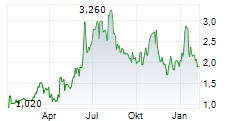
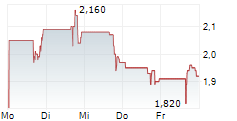
OKYO PHARMA LIMITED gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 1,920 Euro und damit +1,05 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu OKYO PHARMA LIMITED finden Sie auf Wallstreet Online.