- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 17.02. | OS Therapies Discloses 2026 Timeline For Lead Drug Regulatory Filings | 1 | Benzinga.com | ||
| 17.02. | OS Therapies: OS Therapies Provides Global Regulatory Update for OST-HER2 in Recurrent, Fully Resected, Pulmonary Metstatic Osteosarcoma | 348 | Newsfile | Additional forthcoming biomarker data from human trial expected to further characterize immune pathway activation and its relationship to clinical outcomesU.S., U.K. and European osteosarcoma key... ► Artikel lesen | |
| 13.02. | OS Therapies Inc - 8-K, Current Report | 1 | SEC Filings | ||
| 04.02. | OS Therapies: OS Therapies Applauds Reauthorization of Pediatric Priority Review Voucher Program to Advance Breakthrough Osteosarcoma Immunotherapies | 370 | Newsfile | New York, New York--(Newsfile Corp. - February 4, 2026) - OS Therapies Inc. (NYSE American: OSTX) ("OS Therapies" or "the Company"), the world leader in listeria-based cancer immunotherapies, is... ► Artikel lesen | |
| OS THERAPIES Aktie jetzt für 0€ handeln | |||||
| 02.02. | OS Therapies: OS Therapies Initiates US FDA BLA Filing for OST-HER2 in the Prevention or Delay of Recurrent, Fully Resected, Pulmonary Metastatic Osteosarcoma | 369 | Newsfile | Request for FDA Rolling Review submitted to FDA on January 30, 2026Non-Clinical and CMC BLA modules submitted to FDAAt FDA's request, Type D Meeting expected in March 2026 to review Comparative... ► Artikel lesen | |
| 15.01. | OS Therapies Bone Cancer Trial Data Strengthens FDA Case | 3 | Benzinga.com | ||
| 15.01. | OS Therapies: OS Therapies Announces Positive Biomarker Data from Phase 2b Clinical Trial of OST-HER2 in the Prevention or Delay of Recurrent, Fully Resected, Pulmonary Metastatic Osteosarcoma | 160 | Newsfile | Activation of immune blood biomarkers from interferon gamma pathway distinguished long term survivors (>=2 years) from short-term survivors (Pre-specified pathway analysis strategy developed as... ► Artikel lesen | |
| 14.01. | OS Therapies: OS Therapies Announces Filing Form S-1 of OS Animal Health Subsidiary | 410 | Newsfile | OS Animal Health (OSAH), a wholly-owned subsidiary of OS Therapies (OSTX), targeting Initial Public Offering (IPO) on NYSE American or Nasdaq Capital Markets national stock exchange in the first... ► Artikel lesen | |
| 12.01. | OS Therapies: OS Therapies Enters into Warrant Inducement Agreements | 221 | Newsfile | $7.53M gross proceeds raised from pre-existing investors, providing capital runway into 2027All nine investors that were offered agreed to participateNet proceeds to fund OST-HER2 regulatory approval... ► Artikel lesen | |
| 12.01. | OS Therapies Inc - 8-K, Current Report | - | SEC Filings | ||
| 05.01. | OS Therapies provides first half 2026 corporate outlook | 3 | Seeking Alpha | ||
| 05.01. | OS Therapies: OS Therapies Provides First Half 2026 Corporate Outlook | 439 | Newsfile | Company finalizing preparations for end of January 2026 U.S. FDA Biologics License Application (BLA) submission for OST-HER2 program in the prevention or delay of recurrent, fully resected, pulmonary... ► Artikel lesen | |
| 09.12.25 | OS Therapies Announces Successful pre-Marketing Authorisation Application Meeting with UK MHRA Regarding the Phase 2b Clinical Trial of OST-HER2 in the Prevention or Delay of Recurrent, Fully Resected, Pulmonary Metastatic Osteosarcoma | 385 | Newsfile | Alignment achieved on all key points surrounding non-clinical, CMC and post-market authorization confirmatory study designBiomarker data advanced as key pre-specified surrogate clinical efficacy endpoint... ► Artikel lesen | |
| 05.12.25 | OS Therapies Announces FDA PDUFA Waiver & EMA Grants Union Marketing Authorisation Eligibility | 511 | Newsfile | U.S. FDA grants waiver of application fee for BLA Filing of OST-HER2Scheduled pre-Marketing Authorisation Application meeting with United Kingdom's Medicines and Healthcare products Regulatory Agency... ► Artikel lesen | |
| 25.11.25 | OS Therapies Receives Non-Proprietary Name 'daznelimgene lisbac' for OST-HER2 from World Health Organization | 382 | Newsfile | New York, New York--(Newsfile Corp. - November 25, 2025) - OS Therapies Inc. (NYSE American: OSTX) ("OS Therapies" or "the Company"), the world leader in listeria-based cancer immunotherapies, today... ► Artikel lesen | |
| 20.11.25 | OS Therapies to spin off OS Animal Health into a standalone public company | 5 | Seeking Alpha | ||
| 20.11.25 | OS Therapies plans to spin off animal health unit as public company | 5 | Investing.com | ||
| 20.11.25 | OS Therapies to Spinoff OS Animal Health into Standalone Public Company | 383 | Newsfile | Successful preliminary discussion with NYSE representatives and potential investorsOS Therapies shareholders to receive direct equity participation in new listing'Shelter Me: Cancer Pioneers' film... ► Artikel lesen | |
| 17.11.25 | OS Therapies: Aktie fällt nach höherem Quartalsverlust im Zuge von Zulassungsvorbereitungen | 16 | Investing.com Deutsch | ||
| 17.11.25 | OS Therapies GAAP EPS of -$0.21 misses by $0.08 | 5 | Seeking Alpha |
1 2 3 Weiter >>
47 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| CRISPR THERAPEUTICS | 49,000 | -1,61 % | Is CRISPR Therapeutics Stock Too Risky to Buy Right Now? | ||
| IMMUNIC | 0,942 | +1,73 % | EQS-News: Immunic AG: Immunic to Participate in Investor Conferences in March | Issuer: Immunic AG
/ Key word(s): Conference
Immunic to Participate in Investor Conferences in March
03.03.2026 / 12:30 CET/CEST
The issuer is solely responsible for... ► Artikel lesen | |
| PHIO PHARMACEUTICALS | 1,020 | 0,00 % | Quantum XChange treibt mit der neuesten Version von Phio TX einen EUCC-Meilenstein und die Skalierbarkeit für Unternehmen voran | Neuer EUCC-Compliance-Modus und bessere Hive-Architektur stärken die Sicherheit für regulierte europäische und globale Netzwerke Quantum XChange hat heute die neueste Version seiner Kryptografie-Managementplattform... ► Artikel lesen | |
| ANAVEX LIFE SCIENCES | 3,908 | -3,32 % | ANAVEX LIFE SCIENCES CORP in abwartender Struktur | ||
| ARCTURUS THERAPEUTICS | 8,350 | 0,00 % | Arcturus outlines 12-week Phase II CF trial launch and expects regulatory clarity for ARCT-810 in 2026 | ||
| ZEVRA THERAPEUTICS | 7,400 | -3,90 % | Zevra Therapeutics Announces Details for Q4 and Full Year 2025 Financial Results Call | ||
| PROTALIX BIOTHERAPEUTICS | 2,400 | -1,64 % | Chiesi Global Rare Diseases and Protalix BioTherapeutics Receive Positive CHMP Opinion for an Additional Dosing Regimen of Every Four Weeks for Elfabrio (pegunigalsidase alfa) in the EU | Committee
for
Medicinal
Products
for
Human
Use
(CHMP)
issues
a
positive
opinion following re-examination, which
will
be reviewed by
the... ► Artikel lesen | |
| GEOVAX LABS | 1,560 | 0,00 % | EQS-News: GeoVax, Inc.: GeoVax Announces Oncology Advisory Board to Advance Gedeptin(R) Development Across Solid Tumors | EQS-News: GeoVax, Inc.
/ Key word(s): Financial
GeoVax Announces Oncology Advisory Board to Advance Gedeptin(R) Development Across Solid Tumors 24.02.2026 / 15:04 CET/CEST
The... ► Artikel lesen | |
| SCILEX | 7,160 | -6,77 % | XFRA NEW INSTRUMENTS AVAILABLE ON 10.02.2026 | The following instruments on XETRA do have their first trading 10.02.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 10.02.2026
Aktien
1 CNE100006V73 Auntea Jenny (Shanghai)... ► Artikel lesen | |
| INVIVYD | 1,720 | 0,00 % | Invivyd Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| MEIRAGTX | 6,500 | +1,56 % | MeiraGTx Reports Third Quarter 2025 Financial and Operational Results | Entered into broad strategic collaboration with Eli Lilly and Company ("Lilly") in the area of ophthalmology, granting Lilly worldwide exclusive rights to the Company's AAV-AIPL1 program for treatment... ► Artikel lesen | |
| TENAYA THERAPEUTICS | 0,553 | -2,93 % | Tenaya Therapeutics, Inc.: Tenaya Therapeutics Announces 2026 Strategic Priorities and Anticipated Milestones | Aims to Build on Positive 2025 Interim TN-201 Results in First Half of 2026 with Longer-Term Follow-Up Data for Cohorts 1 and 2 from MyPEAK-1 Trial of Adults with MYBPC3-Associated HCM Expects to... ► Artikel lesen | |
| UNICYCIVE THERAPEUTICS | 6,990 | 0,00 % | Unicycive Therapeutics, Inc.: Unicycive Therapeutics Announces FDA Acceptance of Oxylanthanum Carbonate (OLC) New Drug Application (NDA) Resubmission | DA assigns Prescription Drug User Fee Act (PDUFA) target date of June 27, 2026Ended 2025 with unaudited cash position of $41.3M with expected runway into 2027 LOS ALTOS, Calif., Jan. 29, 2026 (GLOBE... ► Artikel lesen | |
| BIONTECH | 86,30 | -0,52 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| QIAGEN | 41,105 | +1,00 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
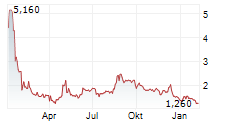
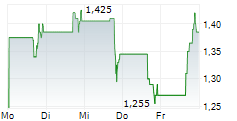
OS THERAPIES INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 1,440 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu OS THERAPIES INC finden Sie auf Wallstreet Online.