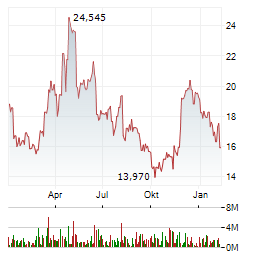
Chart-Typ:
Zeitraum:
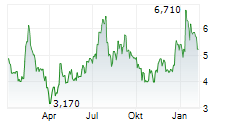
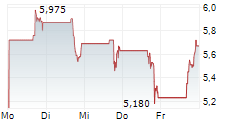
| Erasca, Inc.: Erasca Announces Issuance of a U.S. Patent Covering Pan-KRAS Inhibitor ERAS-4001 | The issued patent provides intellectual property protection for ERAS-4001 and related compositions until at least 2043 Expands Erasca's diversified IP portfolio for RAS-driven cancers Initial Phase... ► Artikel lesen |
| Erasca, Inc.: Erasca Announces Pricing of Upsized Public Offering of Common Stock | SAN DIEGO, Jan. 21, 2026 (GLOBE NEWSWIRE) -- Erasca, Inc. (Nasdaq: ERAS), a clinical-stage precision oncology company singularly focused on discovering, developing, and commercializing therapies for... ► Artikel lesen |
| Erasca, Inc.: Erasca Announces Proposed Public Offering of $150 Million of Common Stock | SAN DIEGO, Jan. 20, 2026 (GLOBE NEWSWIRE) -- Erasca, Inc. (Nasdaq: ERAS), a clinical-stage precision oncology company singularly focused on discovering, developing, and commercializing therapies for... ► Artikel lesen |
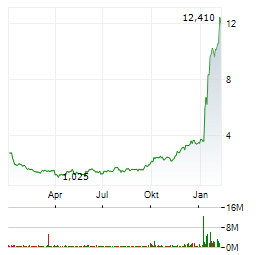
| Dyne Therapeutics, Inc.: Dyne Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Business Highlights | - Planned submission for U.S. Accelerated Approval of z-rostudirsen on track for Q2 2026; potential launch in Q1 2027 - - Positive topline results reported from Phase 1/2 DELIVER trial of z-rostudirsen... ► Artikel lesen |
| Dyne Therapeutics, Inc.: Dyne Therapeutics Receives Orphan Drug Designation in Japan for Zeleciment Basivarsen (DYNE-101) for Myotonic Dystrophy Type 1 | - Zeleciment basivarsen (z-basivarsen) demonstrated sustained functional improvement across multiple clinical measures in the ongoing ACHIEVE clinical trial - - Expect to complete enrollment in ACHIEVE... ► Artikel lesen |
| Dyne Therapeutics, Inc.: Dyne Therapeutics Announces Closing of Upsized Public Offering of Common Stock and Full Exercise by Underwriters of Option to Purchase Additional Shares | WALTHAM, Mass., Dec. 11, 2025 (GLOBE NEWSWIRE) -- Dyne Therapeutics, Inc. (Nasdaq: DYN), a clinical-stage company focused on delivering functional improvement for people living with genetically driven... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
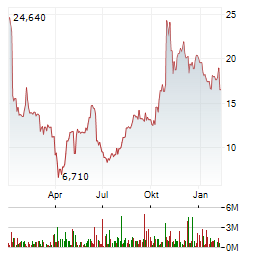
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu FOGHORN THERAPEUTICS INC finden Sie auf Wallstreet Online.