| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 78,50 | 80,00 | 06.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 26.02. | Citizens raises Protagonist Therapeutics stock price target on drug approvals | 1 | Investing.com | ||
| PROTAGONIST THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 25.02. | Protagonist Therapeutics: Protagonist Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Corporate Update | 1.167 | ACCESS Newswire | NDA for rusfertide submitted to the US Food and Drug Administration (FDA), with potential approval and launch this yearCompany expects to opt-out of the 50:50 profit and loss sharing arrangement for... ► Artikel lesen | |
| 25.02. | Protagonist Therapeutics Q4 Earnings Summary & Key Takeaways | 3 | Benzinga.com | ||
| 25.02. | Protagonist Therapeutics, Inc - S-8, Securities to be offered to employees in employee benefit plans | 3 | SEC Filings | ||
| 25.02. | Protagonist Therapeutics, Inc - 10-K, Annual Report | 3 | SEC Filings | ||
| 25.02. | Protagonist Therapeutics, Inc - 8-K, Current Report | 1 | SEC Filings | ||
| 24.02. | Protagonist Therapeutics to Participate in Multiple Investment Bank Conferences in March 2026 | 227 | ACCESS Newswire | NEWARK, CA / ACCESS Newswire / February 24, 2026 / Protagonist Therapeutics, Inc. ("Protagonist" or the "Company") today announced that Dinesh V. Patel, Ph.D., President and Chief Executive Officer... ► Artikel lesen | |
| 11.02. | H.C. Wainwright Raises Protagonist Therapeutics, Inc. (PTGX) Target to $117, Reiterates Buy | 1 | Insider Monkey | ||
| 30.01. | Breaking Down Protagonist Therapeutics: 8 Analysts Share Their Views | 1 | Benzinga.com | ||
| 30.01. | Protagonist Therapeutics stock price target raised to $117 from $80 at H.C. Wainwright | 2 | Investing.com | ||
| 27.01. | JPMorgan reiterates Overweight rating on Protagonist Therapeutics stock | 1 | Investing.com | ||
| 22.01. | ClearBridge Small-Cap Growth trims Duolingo, Biohaven, QLYS; Adds PTGX, SSD, BETA, DYN in Q4 | 26 | Seeking Alpha | ||
| 12.01. | Protagonist Therapeutics, Inc - 8-K, Current Report | - | SEC Filings | ||
| 07.01. | Protagonist Therapeutics to Participate in the 44th Annual J.P. Morgan Healthcare Conference 2026 | 308 | ACCESS Newswire | NEWARK, CA / ACCESS Newswire / January 7, 2026 / Protagonist Therapeutics, Inc. ("Protagonist" or the "Company") today announced that Dinesh V. Patel, Ph.D., President and Chief Executive Officer, will... ► Artikel lesen | |
| 05.01. | Truist raises Protagonist Therapeutics stock price target to $110 on pivotal year ahead | 4 | Investing.com | ||
| 05.01. | Takeda Pharmaceutical, Protagonist Therapeutics Announces Submission Of NDA For Rusfertide | 669 | AFX News | TOKYO (dpa-AFX) - Takeda Pharmaceutical Company Limited (TAK) and Protagonist Therapeutics, Inc. (PTGX), Monday announced the submission of a New Drug Application regarding rusfertide for the... ► Artikel lesen | |
| 12.12.25 | Citizens maintains Protagonist Therapeutics stock rating, citing rusfertide efficacy | 2 | Investing.com | ||
| 27.11.25 | Protagenic Therapeutics Announces Receipt of Nasdaq Non-Compliance Notice | 475 | ACCESS Newswire | NEW YORK, NY / ACCESS Newswire / November 27, 2025 / Protagenic Therapeutics, Inc. (Nasdaq:PTIX) (the "Company") today announced that it has received a notification letter from the Nasdaq Listing Qualifications... ► Artikel lesen | |
| 07.11.25 | Protagonist Therapeutics: Clear Street hebt Kursziel deutlich auf 91 Dollar an | 5 | Investing.com Deutsch | ||
| 07.11.25 | Protagonist Therapeutics stock price target raised to $91 at Clear Street | 2 | Investing.com |
1 2 3 4 Weiter >>
65 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 87,05 | -0,51 % | BioNTech-Konkurrent Moderna: Satter Kurssprung - jetzt noch einsteigen? | Die Aktie von Moderna hat zuletzt erneut kräftigen Rückenwind erhalten. Die Korrektur seit Ende Januar wurde damit nicht nur vollständig wieder ausgeglichen, das Papier konnte sogar auf ein neues Jahreshoch... ► Artikel lesen | |
| EVOTEC | 5,402 | -1,13 % | Shortseller-Positionen aktuell: Evotec, Gerresheimer, HelloFresh, Renk, Hypoport, Symrise, TUI | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| BB BIOTECH | 49,800 | -0,60 % | Christian Koch, Head BB Biotech: «Opportunitäten entstehen dort, wo Fortschritt und Bewertung noch nicht im Gleichgewicht sind» | ||
| MEDIGENE | 0,034 | -11,86 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 39,095 | -3,59 % | Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 45,130 | -0,29 % | Moderna freut sich über eine Zulassungsempfehlung der EMA für einen Kombi-Impfstoff gegen Corona und Grippe | Bei Corona-Impfstoffen war der US-Konzern Moderna noch ein Nachzügler. Anders scheint es nun bei Kombi-Impfstoffen zu laufen, die gleichzeitig vor Corona und der Grippe schützen. Dafür gibt es bisher... ► Artikel lesen | |
| PAION | 0,061 | -21,54 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| AMGEN | 316,00 | -0,74 % | Leerink Partners senkt Amgen-Prognose für Q1 wegen Lagerbestandsabbau | ||
| NOVAVAX | 8,567 | -0,22 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| BIOGEN | 157,65 | -1,00 % | Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen | |
| BIOFRONTERA | 2,680 | +2,68 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 3,030 | -2,26 % | EQS-Adhoc: Heidelberg Pharma AG: Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von Soleus Capital bekannt | EQS-Ad-hoc: Heidelberg Pharma AG / Schlagwort(e): Bedeutende Verträge
Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von... ► Artikel lesen | |
| ILLUMINA | 107,60 | +0,19 % | Illumina (ILMN) Gaining from Stronger Than Expected Results | ||
| CRISPR THERAPEUTICS | 48,200 | -1,23 % | Forget CRISPR Therapeutics: This Gene-Editing Player Already Boasts the Profits It Dreams Of | ||
| NANOREPRO | 1,510 | -1,95 % | NanoRepro: "Kaltes Plasma stärkt die Haut" | NanoRepro setzt mit dem Marktstart ihres Kaltplasmageräts PHLAS ein strategisches Signal: Weg von einmaligen Sondereffekten, hin zu planbaren, margenstarken Erlösmodellen im Health- und Lifestyle-Segment.... ► Artikel lesen |
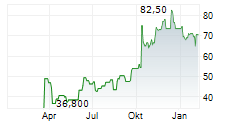
PROTAGONIST THERAPEUTICS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 79,50 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PROTAGONIST THERAPEUTICS INC finden Sie auf Wallstreet Online.