TOKYO (dpa-AFX) - Takeda Pharmaceutical Company Limited (TAK) and Protagonist Therapeutics, Inc. (PTGX), Monday announced the submission of a New Drug Application regarding rusfertide for the treatment of adults with polycythemia vera.
The submission is backed by the positive 32-week primary analysis and 52-week results from the Phase 3 VERIFY study, as well as the Phase 2 REVIVE study.
As per an earlier agreement between the companies, the submission of the NDA triggers the start of a 120-day period, after which Protagonist can decide to exercise its opt-out right during a subsequent 90-day period.
If Protagonist opts out, then it would be eligible for upto $400 million in opt-out payments as well as enhanced milestone payments and 14-29 percent tiered royalty rates on worldwide net sales of rusfertide.
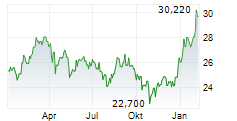
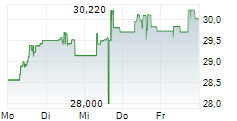
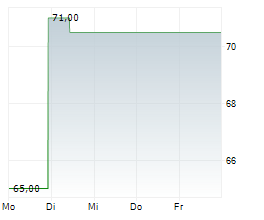
In the pre-market hours, TAK is trading at $15.60, down 0.13 percent on the New York Stock Exchange, and PTGX is trading at $87.50, up 0.37 percent on the Nasdaq.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News